Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
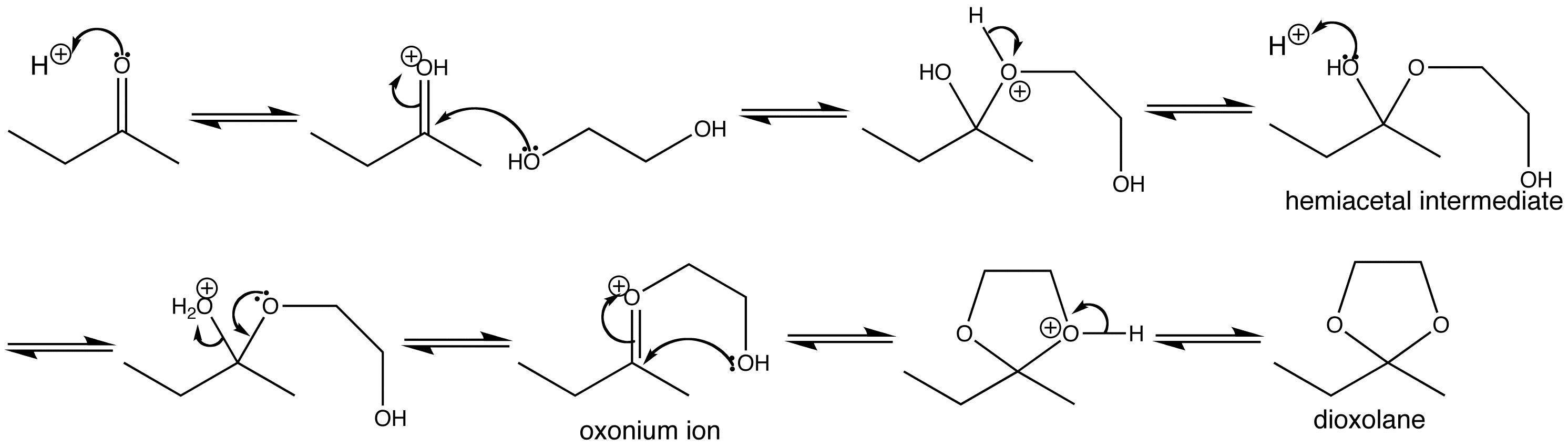
Cyclic acetals are more stable towards hydrolysis than acyclic ones, they are also much easier to make. Cyclic acetals are readily formed by the reaction of two molecules, a ketone and a diol. The reaction produces two products, the acetal plus water, so the usually unfavourable entropy of acetal formation is not a factor. Formation is also kinetically favoured because the intramolecular ring-closing reaction is fast.
S. Krompiec, M. Penkala, K. Szczubiałka and E. Kowalska, Coord. Chem. Rev., 2012, 256, 2057–2095.