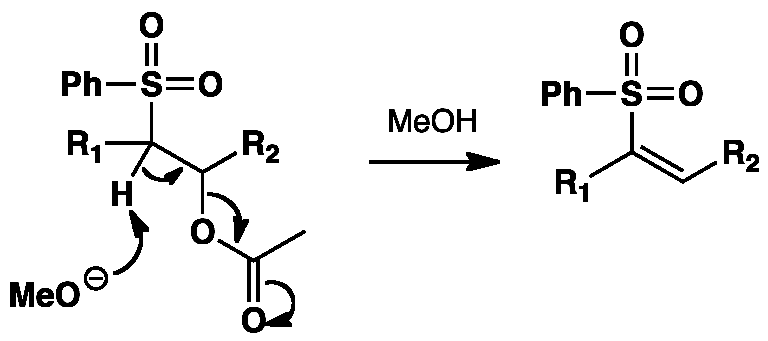
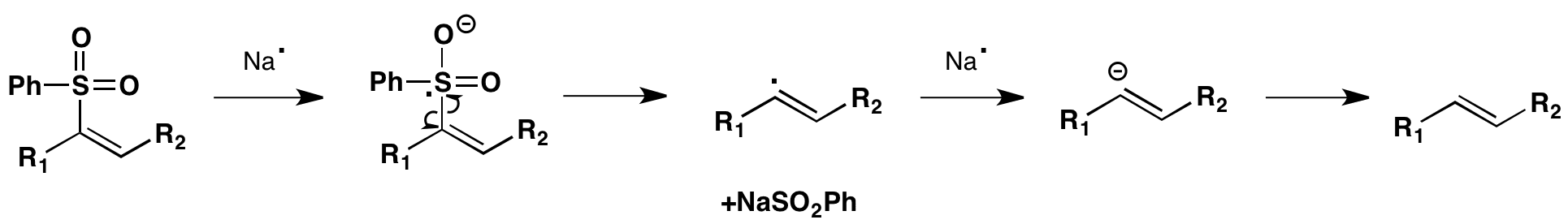
Click the structures and reaction black arrows in sequence to view the 3D models and animations respectively
NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition state phase.
Initial base promoted elimination of carboxylate leads to a vinyl sulphone which is reductively cleaved to a vinyl radical. The stereoselectivity of the Julia olefination is determined by the radical intermediate. The intermediates, both the cis and trans, can equilibrate allowing the more thermodynamically stable E alkene to be produced.
P. R. Blakemore, J. Chem. Soc. Perkin Trans. 1, 2002, 0, 2563–2585.