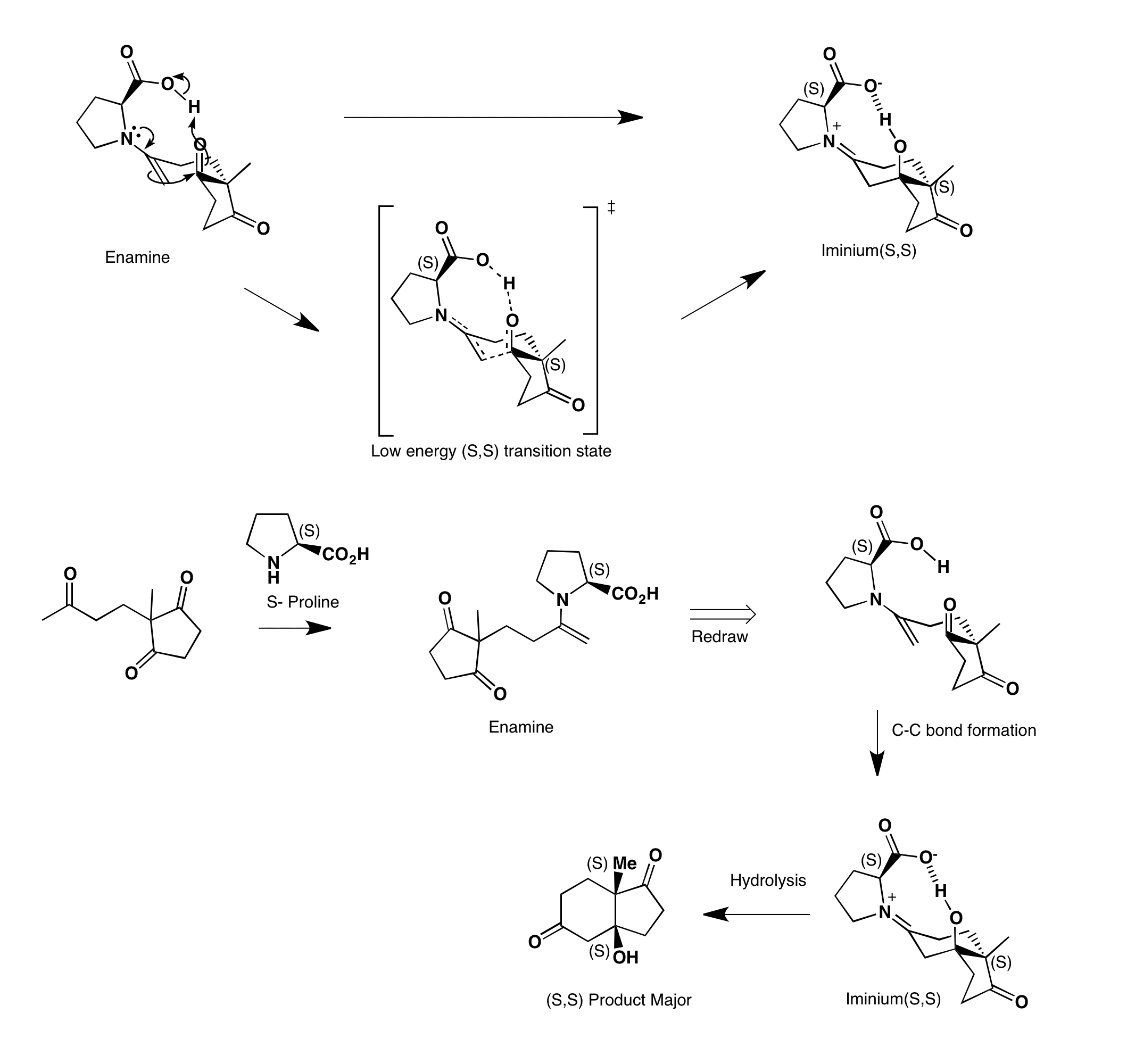
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
Enantioselective intramolecular aldol cyclisations can be catalysed by proline. The first step of the reaction is enamine formation with S-proline and the ketone. Aldol cyclization occurs via a very stable (S,S) transition state. This cyclisation determines the stereochemistry of the product, i.e. the reaction is enantioselective. This forms iminium (S,S), which is then hydrolysed to form the product and releasing proline to act substoichiometrically.
F. R. Clemente and K. N. Houk, Angew. Chemie Int. Ed., 2004, 43, 5766–5768.