Click the Image to view 3D Mechanism
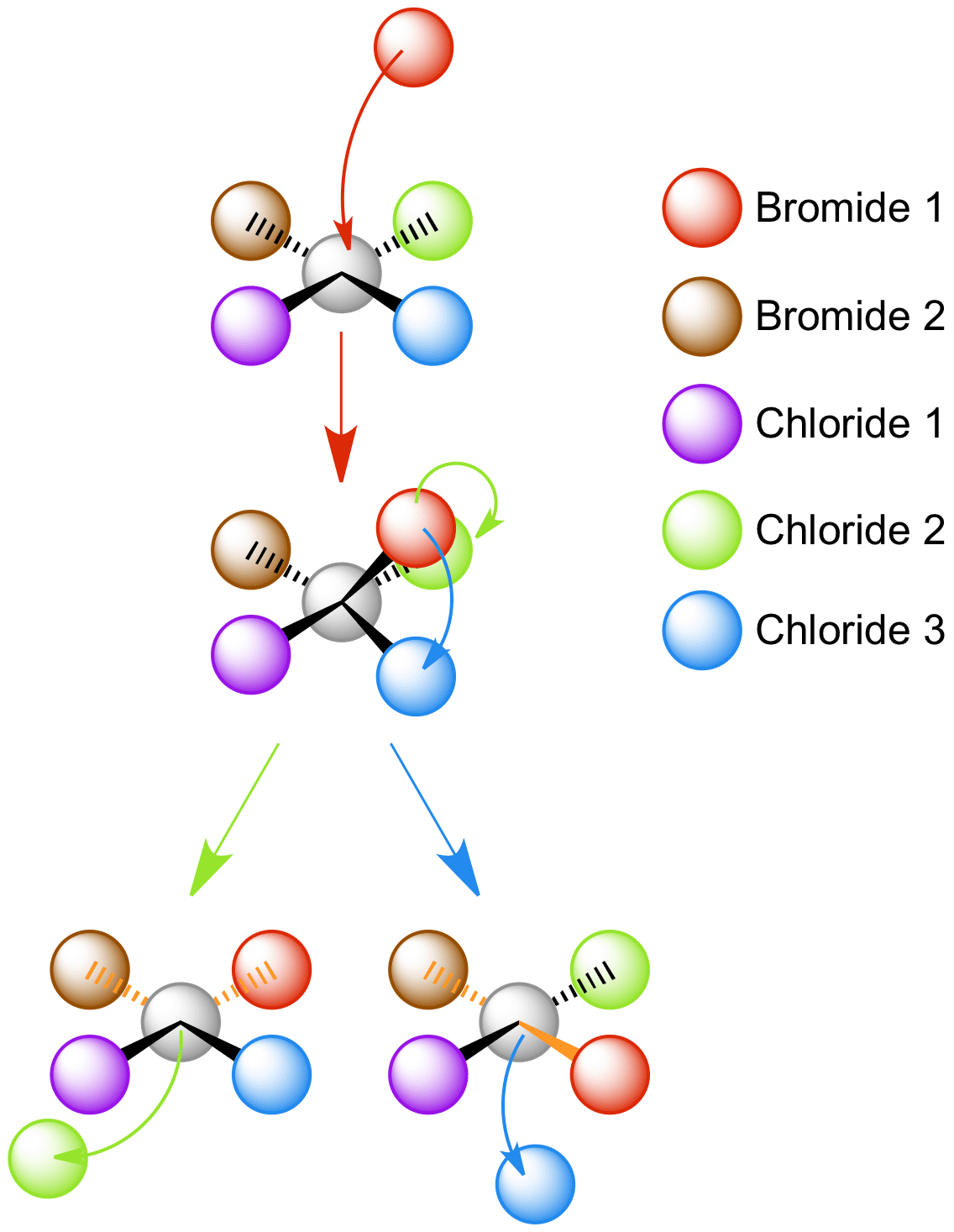
Bromide 1 approaches from the top of the square planar complex, forming the trigonal bypyramidal intermediate. This intermediate can form a cis-[PtCl2Br2]2- or trans-[PtCl2Br2]2- depending on which chloride ligand is removed.
Removal of chloride 2 results in formation of the cis complex, indicated by the 90o orange Br-Pt-Br bonds. Removal of chloride 3 results in formation of the trans complex, indicated by the straight orange Br-Pt-Br bonds.
Inner Sphere Mechanism for Electron Transfer