NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures and reaction arrows to view the 3D models and animations respectively
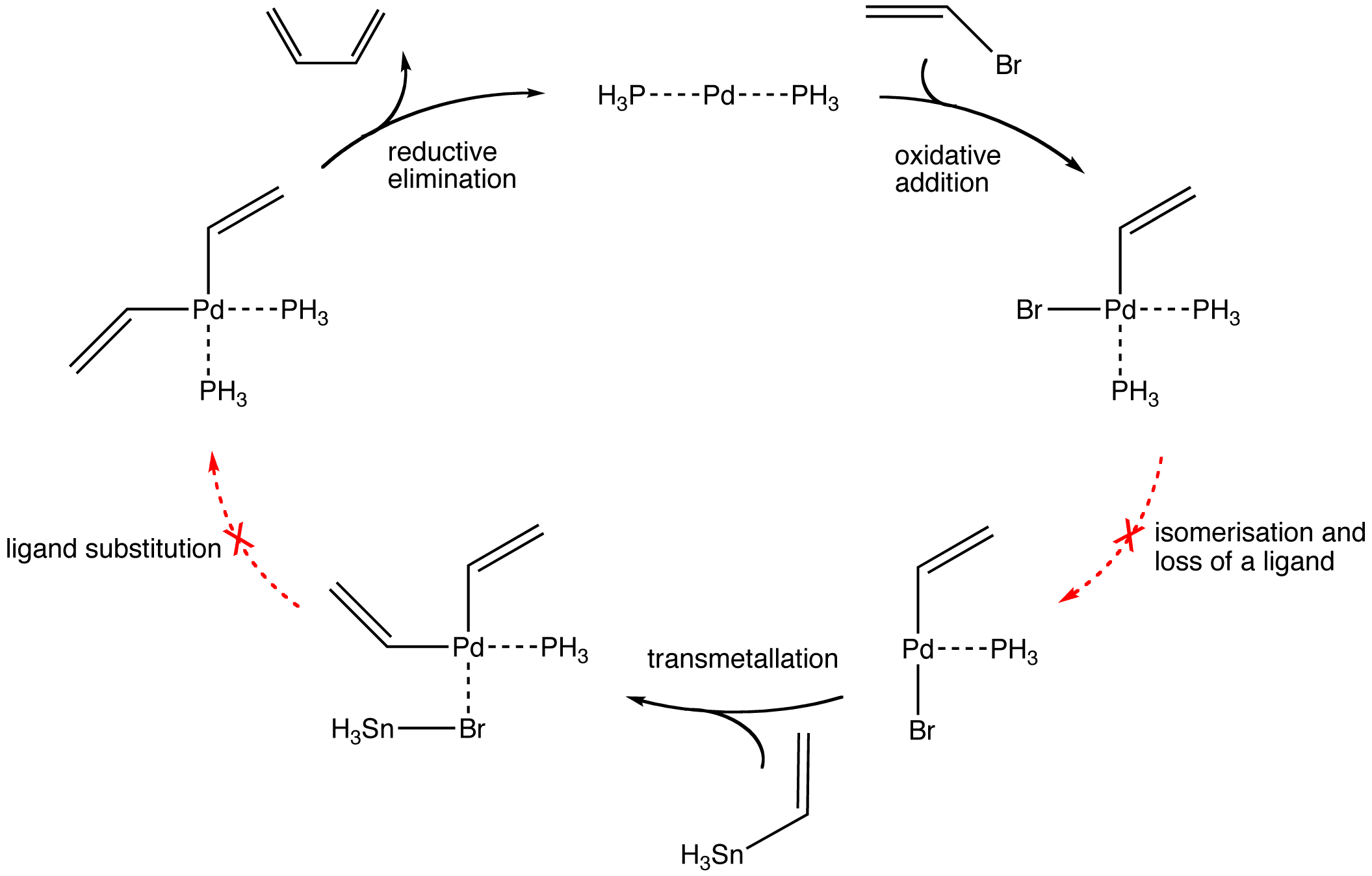
Cis-trans isomerisation and solvent reorganisation throughout the reaction are not shown explicitly.
For simplicity animations are shown with PH3 and SnH3 instead of PR3 and SnR3 respectively.
The Kosugi-Migita-Stille reaction, commonly known as the Stille coupling, is the palladium-catalysed coupling of aromatic and vinylic systems using organostannanes.
The mechanism involves the oxidative addition of vinyl or aromatic triflate or halide to give a palladium intermediate, which undergoes a transmetallation reaction (rate-determining step) with the organostannane to give an organopalladium intermediate. This complex then undergoes a reductive elimination step, giving the product and regenerating the palladium(0) catalyst.
Animations based on R. Álvarez, O.N. Faza, A.R. de Lera, D.J. Cárdenas, Adv. Synth. Catal. 349 (2007) 887-906.
C. Cordovilla, C. Bartolomé, J. M. Martínez-Ilarduya and P. Espinet, ACS Catal., 2015, 5, 3040–3053.