Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
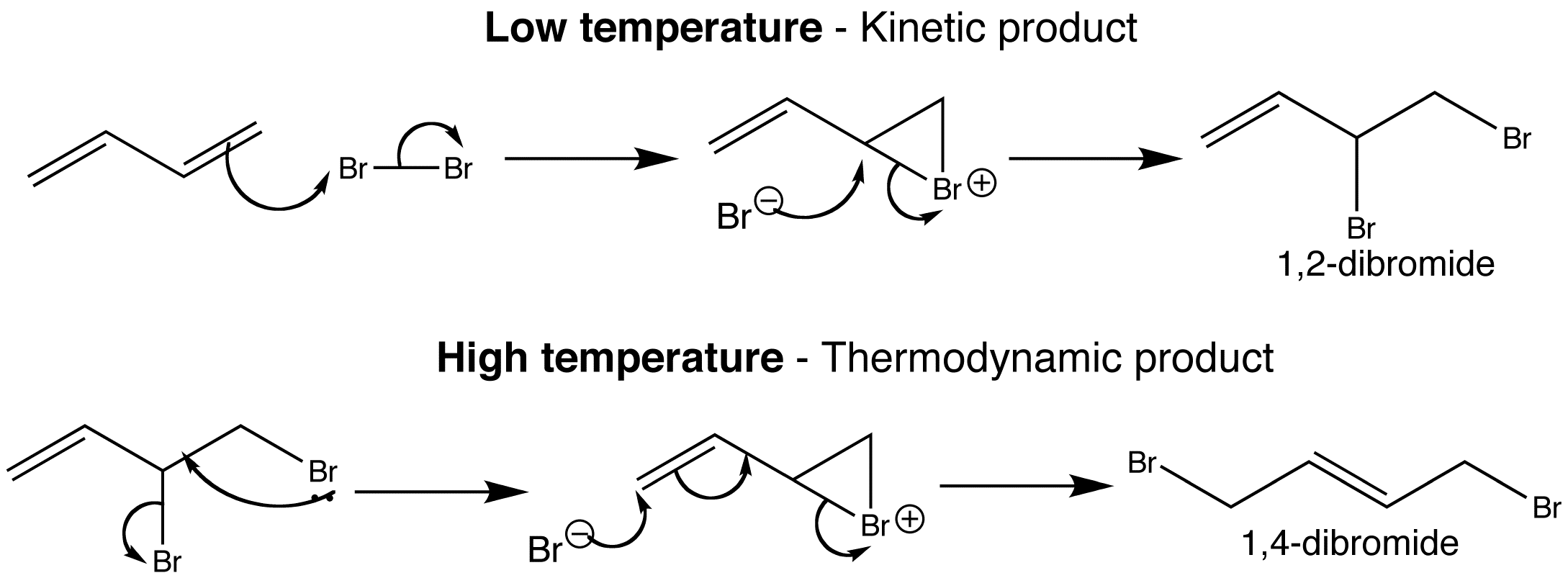
The reaction of bromine with dienes can produce regioisomers; the outcome can be altered depending on the conditions used. If the reaction is done at lower temperatures, the bromine just adds across one of the double bonds to give a 1,2-dibromide. This is the kinetic product.
The 1,4-dibromide is only formed when the reaction is heated and is the thermodynamic product. The mechanism is the electrophilic attack on the diene to give a bromonium ion, which bromide opens to give the dibromide. 1,2-dibromide can then react further because it can undergo nucleophilic substitution.
Bromide is a good nucleophile and leaving group, and with an allylic system like this, SN1 can take place in which both the nucleophile and the electrophile are bromine. Bromide can attack where it left or at the far end of the allylic system, giving the 1,4-dibromide which is more stable.
L. F. Hatch, P. D. Gardner and R. E. Gilbert, J. Am. Chem. Soc., 1959, 81, 5943–5946.