Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
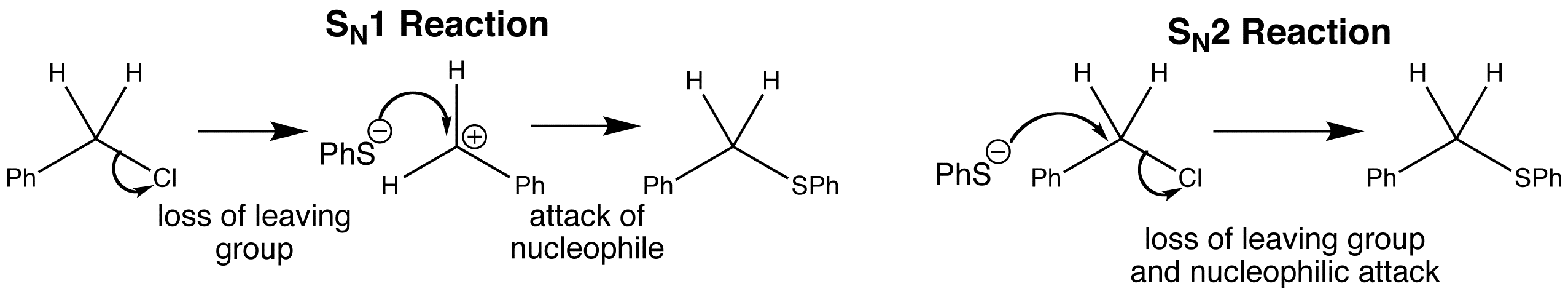
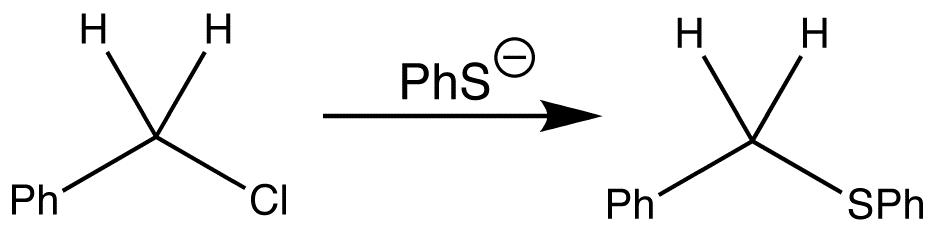
Nucleophilic substitution can occur at saturated carbon atoms. One such reaction is shown below. During the reaction, the phenyl group remains the same and so does the CH2 group, but the Cl group is replaced by the PhS group.
The reaction occurs at the CH2 group, so the reaction is a ‘nucleophilic substitution at a saturated carbon atom’. The electrons from the nucleophile cannot be added to the fully saturated CH2 group, so it is not possible for the nucleophile to add first and the leaving group to go later, as this would give a 5-valent carbon atom. This deduction results in two new and different mechanisms to become possible. Firstly SN1 which involves the loss of the leaving group first followed by the attack of the nucleophile, and secondly an SN2 reaction, where the loss of the leaving group and the attack of the nucleophile happens at the same time. It is only possible for the carbon atom to accept electrons if it loses some either before or at the same time as the nucleophile attacks. Both reactions are possible with this example.
For “Animated Molecular Orbitals – Sn2” –SH was used instead of –SPh. The reaction would proceed in the same way,–SH was used for simplicity
Alternatively, use these links: (Substrate: Nucleophile)
Allyl chloride : SH | Benzyl chloride : SH | 2o benzyl chloride: SH | 2o allyl chloride : SH (SN2′)
E. D. Hughes, Trans. Faraday Soc., 1941, 37, 603.
A. R. Katritzky and B. E. Brycki, J. Phys. Org. Chem., 1988, 1, 1–20.