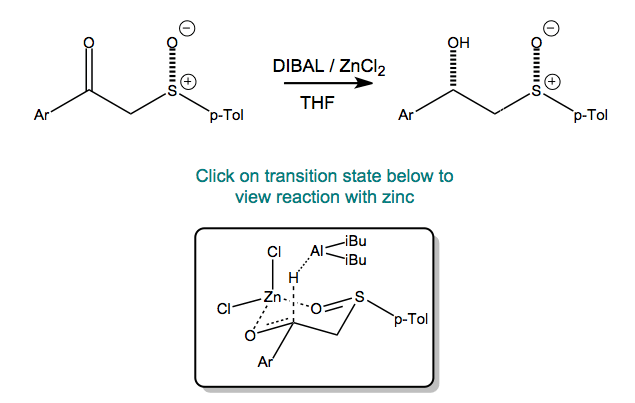
The zinc chloride chelates between the sulfoxide and the ketone. The conformation is a six-membered chair in which the p-toluyl group is pseudo-equatorial. Axial attack of DIBAL leads to the observed stereochemistry.
The stereochemistry of the reaction is controlled by the sulfoxide. In the presence of ZnCl2, the hydride transfer occurs intermolecularly yielding the R,R enantiomer.
Summary page : Without ZnCl2 : With ZnCl2
A. Solladié-Cavallo, J. Suffert, A. Adib and G. Solladié, Tetrahedron Lett., 1990, 31, 6649–6652.