Use the buttons to display the dipoles, charges and electrostatic potential mapped onto the surface of each molecule. Red regions are nucleophilic (electron rich) and blue regions are electrophilic (electron poor)
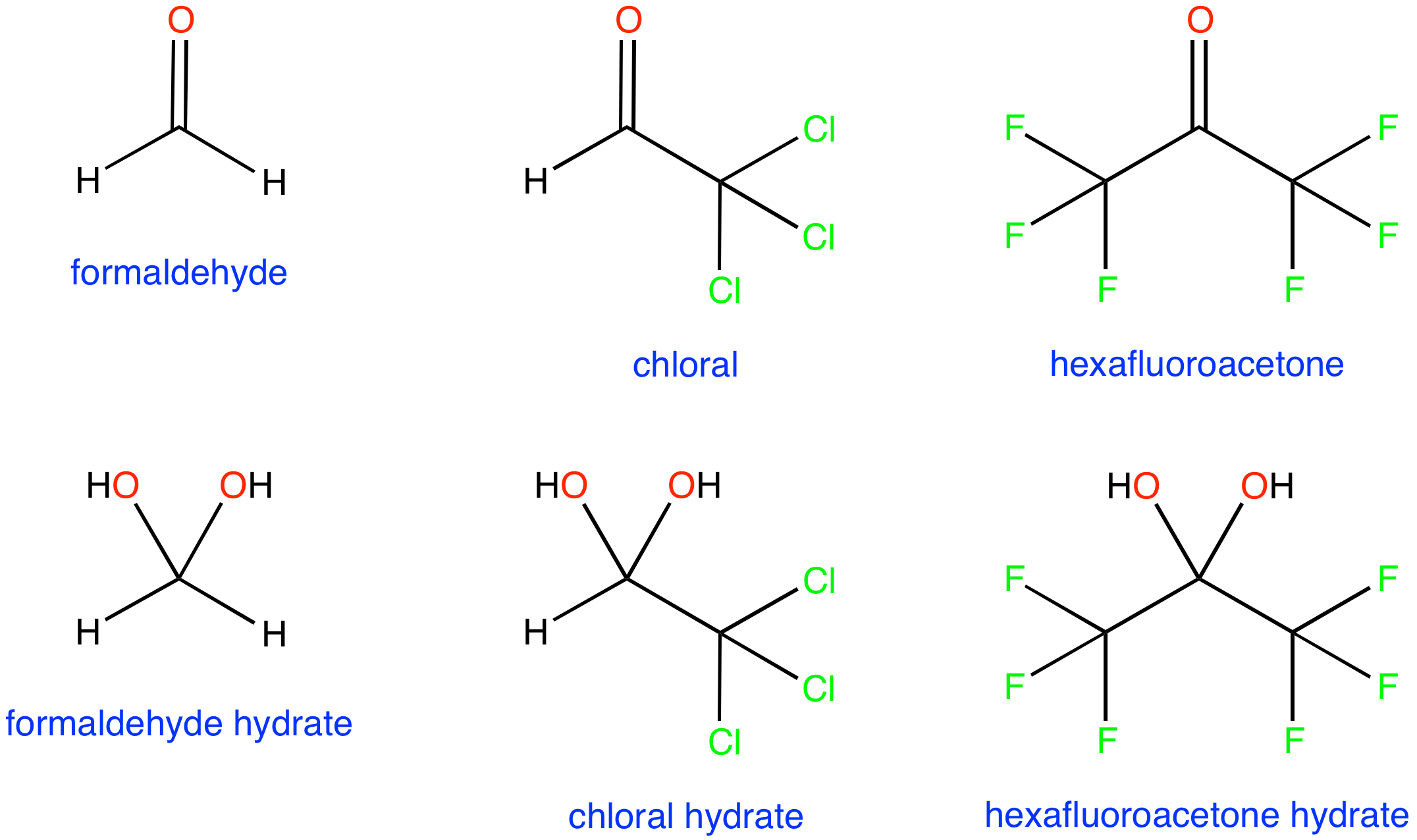
More molecules: Small Polar | Conjugated | Lone Pair Conjugation | Carbonyl Hydration