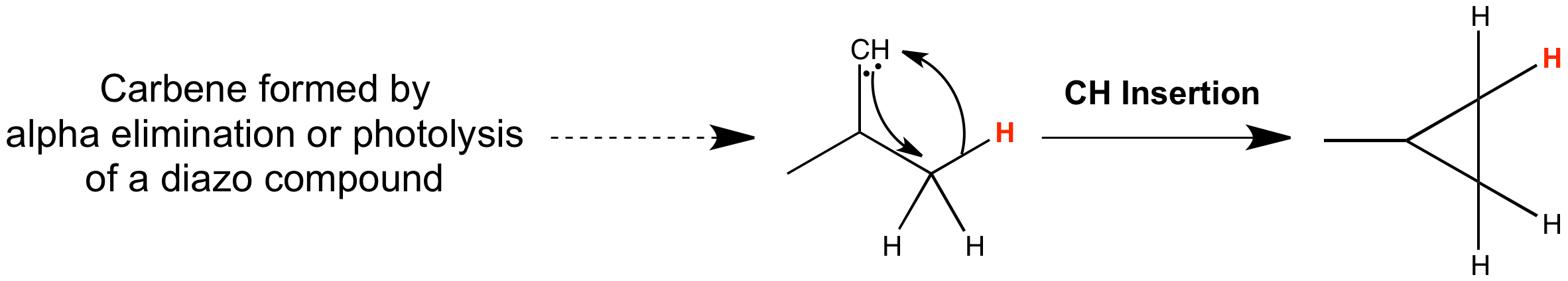
Simple C-H insertion:
(Showing the transition state geometry)
NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
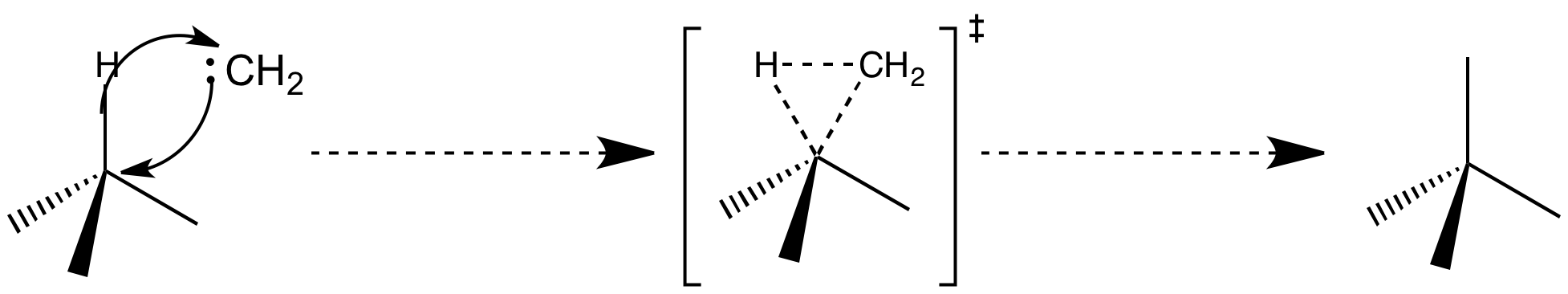
Intramolecular C-H insertion:
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
Carbenes can undergo insertion into a C-H bond. These insertion reactions create new bonds at completely unfunctionalized centres so can be very useful in synthesis. The similarity with cyclopropane formation by intramolecular cycloadditions to alkenes is clear, and the mechanisms mirror one another quite closely. The path of the reaction differs according to whether the carbene is a singlet or triplet. Singlet carbenes can insert in a concerted manner, with the orbitals overlapping constructively provided the carbene approaches side-on, whereas triplet carbenes insert via a two-step radical pathway. However, very few triplet carbene insertions have been observed.
Back to carbene reactions main page
P. de Frémont, N. Marion and S. P. Nolan, Coord. Chem. Rev., 2009, 253, 862–892.