Click the reaction arrow in sequence to view the 3D models and animations respectively
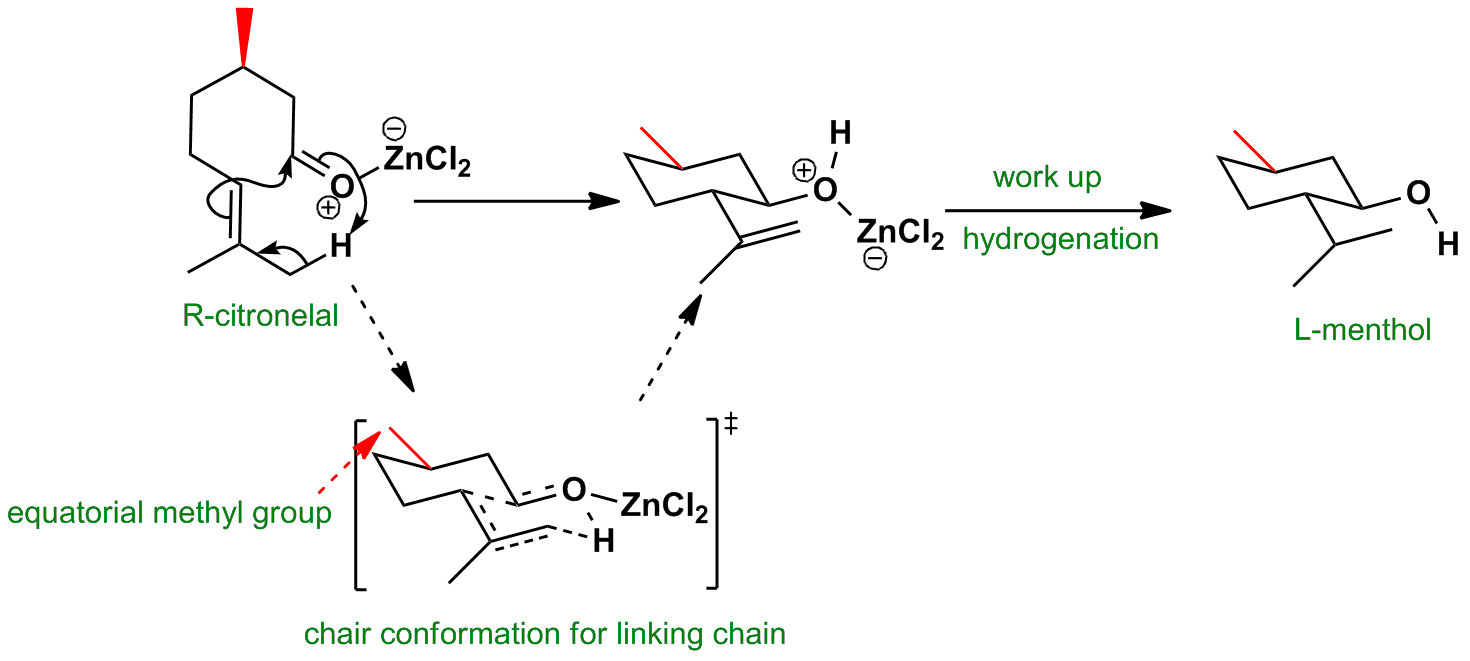
Zinc chloride promotes the cyclisation of R-citronellal and hydrogenation gives L-menthol.
The red methyl group prefers to be equatorial in the transition state and directs the formation of the two new chiral centres. The transition state is like a trans-decalin with two fused six-membered chair rings. Both new substituents go equatorial in the product while the Lewis acid binds to the oxygen and accelerates the reaction.
M. Emura and H. Matsuda, Chem. Biodivers., 2014, 11, 1688–1699.