Click each of the reaction schemes below to view the 3D models and animations respectively
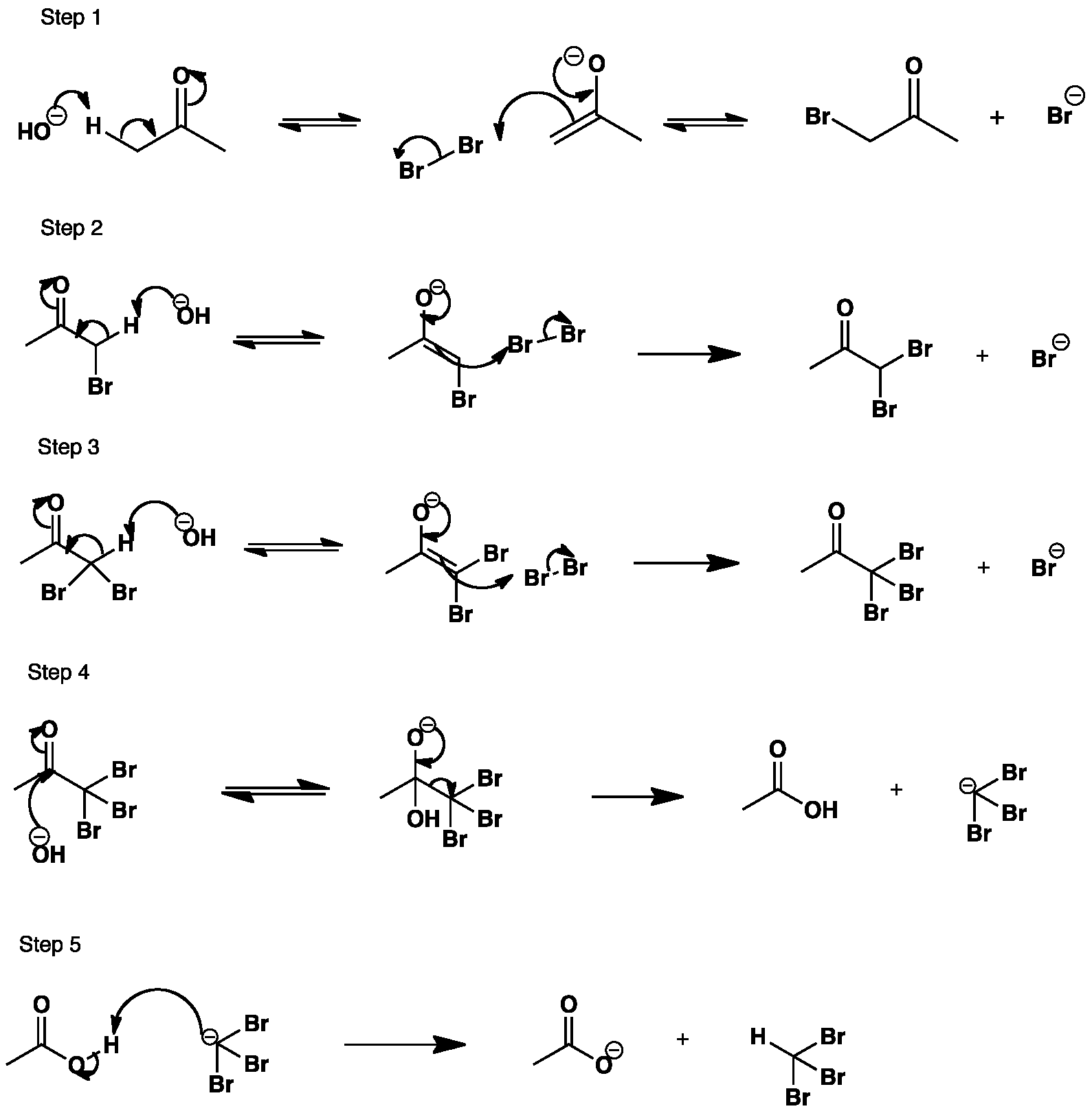
The hydroxide removes a proton from the ketone to form an enolate anion. The enolate anion attacks the bromine molecule yielding a mono-substituted bromoketone. The reaction continues until the tribromoketone is formed. The hydroxide then attacks directly at the carbonyl and a tribromomethyl anion is lost.
E. Tapuhi and W. P. Jencks, J. Am. Chem. Soc., 1982, 104, 5758–5765.