Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
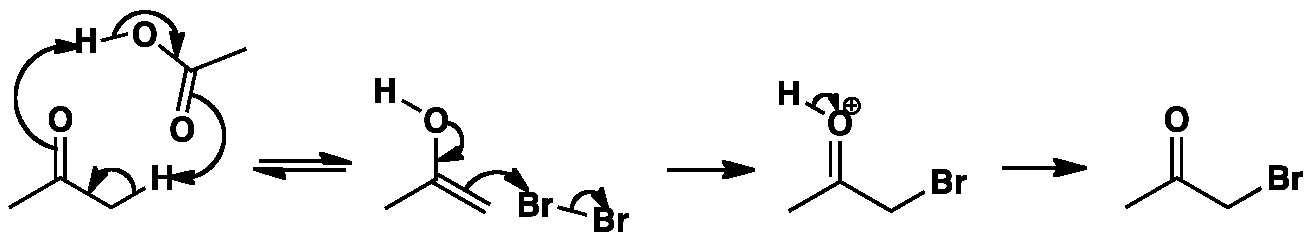
Bromination of ketones occurs smoothly with bromine in acetic acid. The first step is enol formation which can be stepwise or concerted as shown here. Protonation of the carbonyl and formation of the enol occurring at the same time. The next step is the attack of the enol on the bromine. The proton on the carbonyl is then lost to yield bromoacetone.
M. F. Ruasse, in Advances in Physical Organic Chemistry, 1993, vol. 28, pp. 207–291.