Lithium and magnesium are very electropositive metals. The Li-C or Mg-C bonds in organolithium or organomagnesium reagents are highly polarized towards carbon.
Red represents areas which are electron rich, blue represents areas that are electron poor
Organometallics are therefore very powerful nucleophiles, and attack at the carbonyl group to give alcohols, forming a new C-C bond. The reactions from these two classes of organometallic reagents with carbonyl compounds, are among the most important ways of making carbon-carbon bonds.
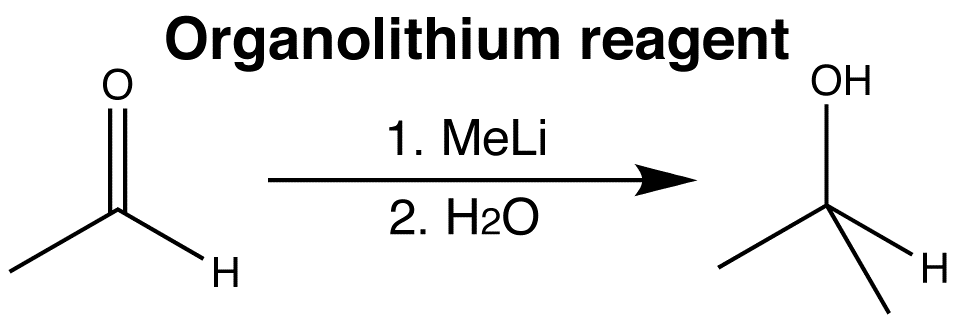
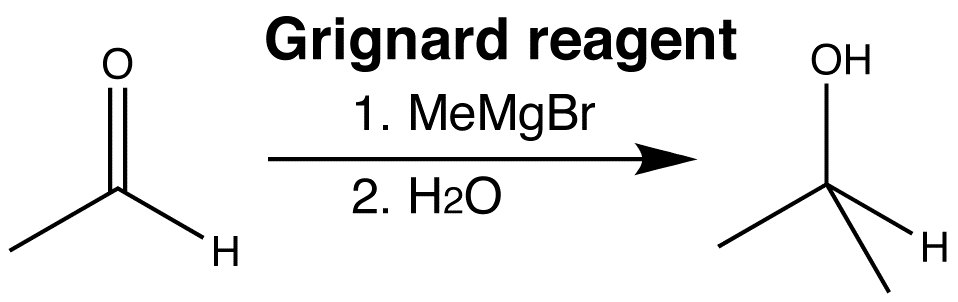
Click the images to view the 3D animations for the two different organometallic reactions
E. C. Ashby, J. Laemmle and H. M. Neumann, Acc. Chem. Res., 1973, 7, 272–280.