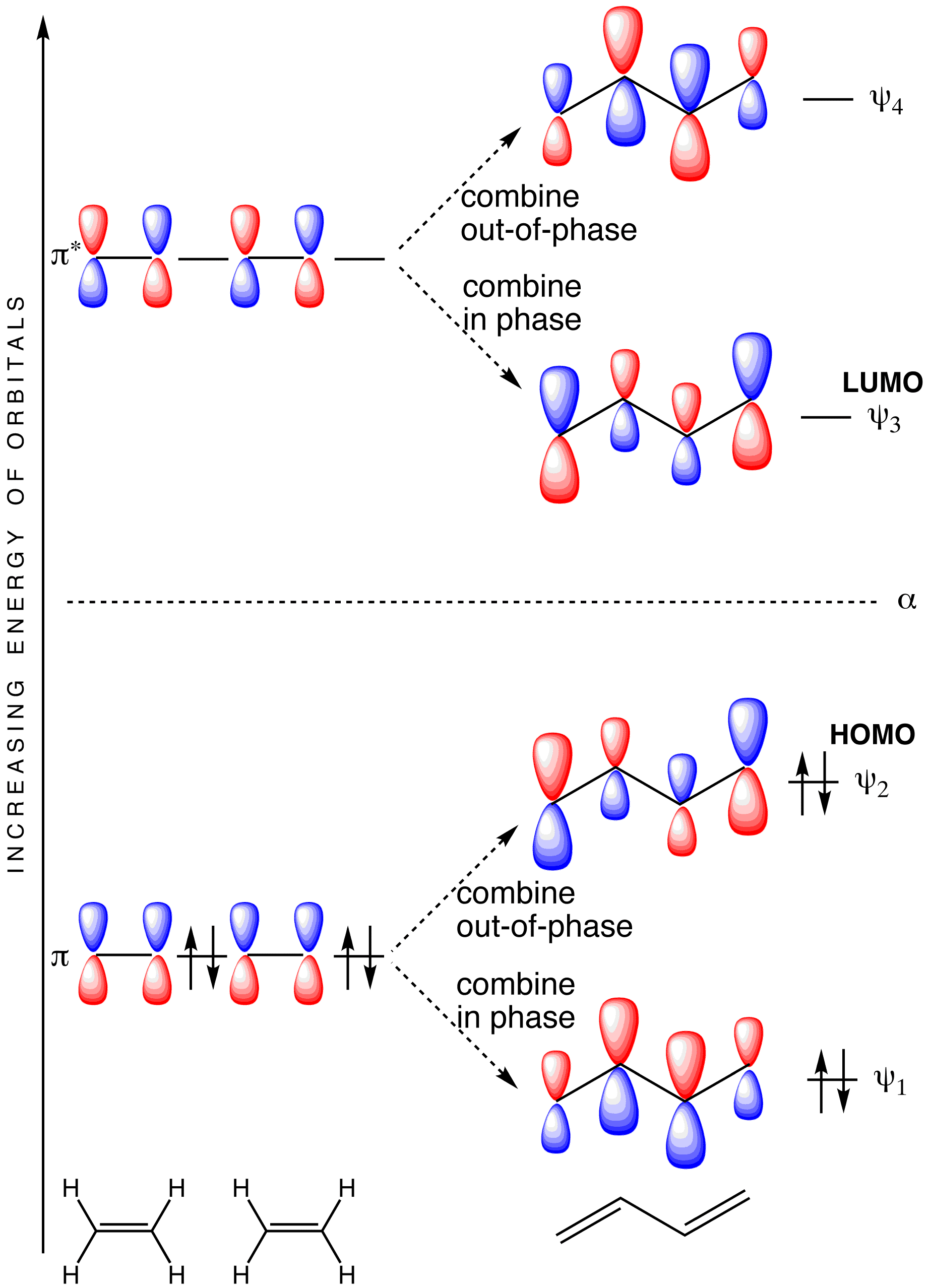
Click on the diagram to display the various bonding and antibonding molecular orbitals.
The molecular orbital diagram for the π-molecular orbitals of butadiene as a result of combining the π-molecular orbitals of two ethene molecules. This shows the effect of conjugation.
•The overall energy of the two bonding butadiene molecular orbitals is lower than that of the two molecular orbitals for ethene. This means that butadiene is more thermodynamically stable than we might expect if its structure were just two isolated double bonds
•The HOMO for butadiene is higher in energy relative to the HOMO for ethene. This means butadiene should be more reactive than ethene towards nucleophiles
•The LUMO for butadiene is lower in energy than the LUMO for ethene. Consequently, butadiene would be expected to be more reactive towards nucleophiles than ethene
•So whilst butadiene is more stable than two isolated double bonds, it is also more reactive
Explore bonding orbitals in other small molecules
Hydrogen | Fluorine | Nitrogen | Hydrogen Fluoride | Carbon Monoxide | Methane | Ammonia | Ethylene | Acetylene | Allene | Formaldehyde | Benzene