Click the reaction arrow in sequence to view the 3D models and animations respectively
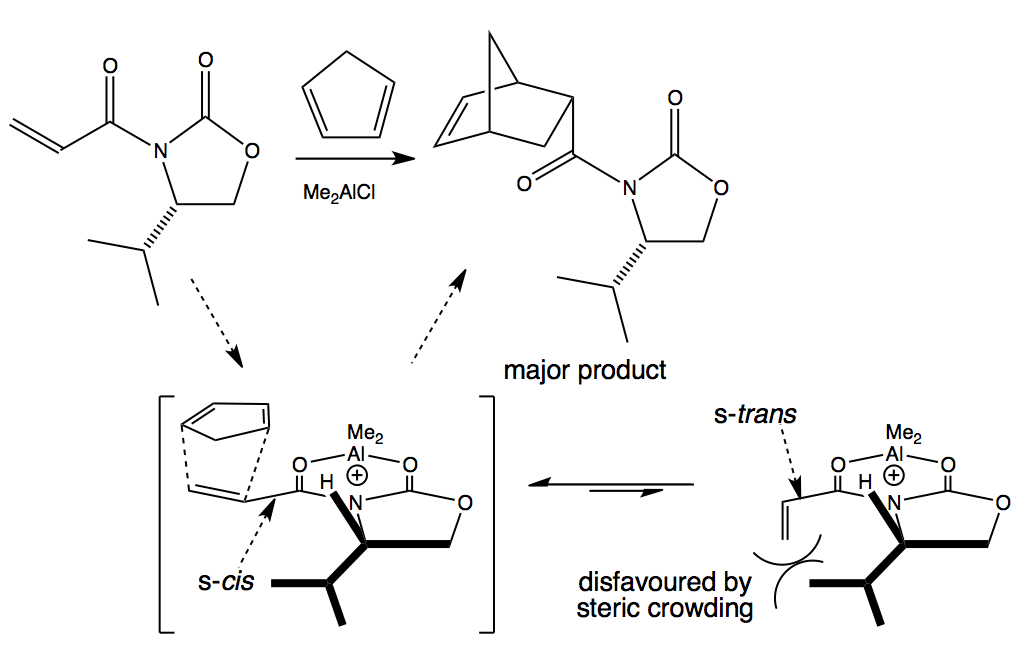
The Lewis acid chelates between the carbonyl groups of the dienophile which activates the alkene and locks the conformation so that the bulky isopropyl group blocks one face of the system in the s-cis conformation. Cyclopentadiene undergoes Diels-Alder reaction on the less hindered face.
D. A. Evans, K. T. Chapman and J. Bisaha, J. Am. Chem. Soc., 1988, 110, 1238–1256.