NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
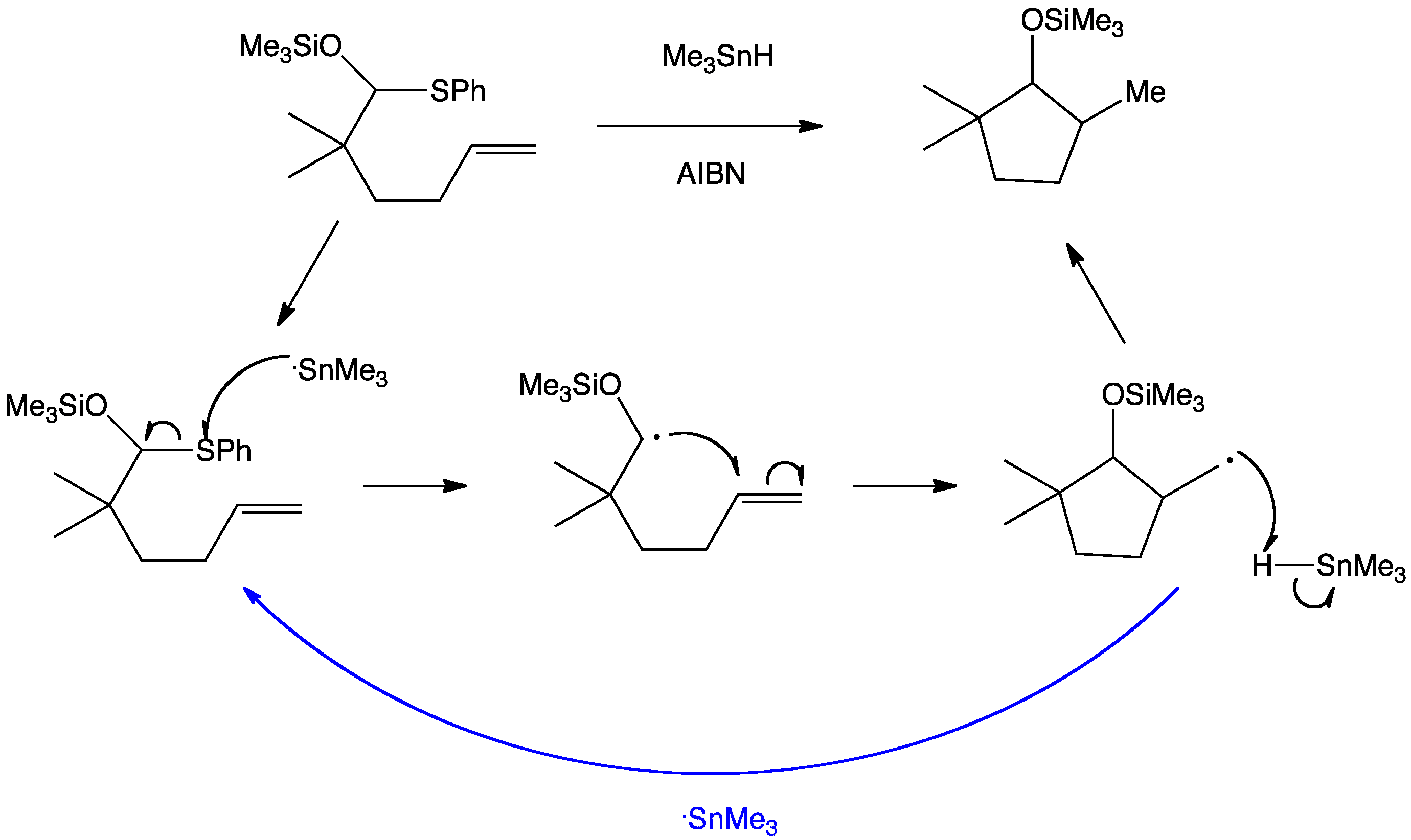
The reaction is initiated much in the same way as the “SnMe3” radical chain whereby azobis(isobutyronitrile) homolyses to form two 2-cyanoprop-2yl radicals and nitrogen gas. As the alkene is not activated in this case the radical prefers to attack the weak carbon to sulfur bond. The resultant alkyl radical is stabilised by interactions with the oxygen lone pair raising the SOMO’s energy. Cyclisation is now favoured as the alkene double bond is held close to the alkyl radical. Finally abstraction of a hydrogen from tin hydride leads to the product and the tin radical carries the chain.
B. Giese, Angew. Chemie Int. Ed. English, 1985, 24, 553–565.