Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
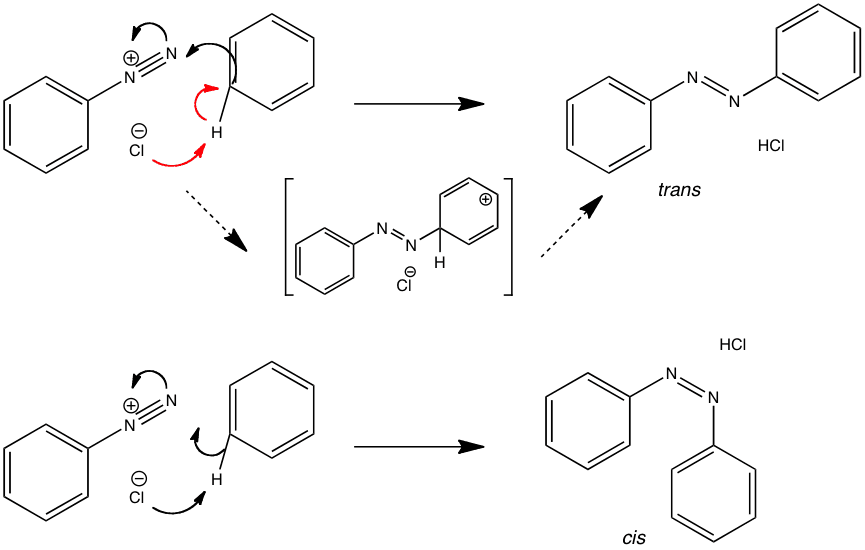
The coupling reaction between benzene and the diazonium salt was modelled using Spartan ’08 software to produce some interesting results.
Although both reactions appear to be “single step”, the mechanism of the trans isomer displays an initial addition reaction followed by a distinct deprotonation step (red arrows). In the mechanism of the cis isomer, it can be seen that these steps occur simultaneously.
Substituents on the benzene, usually at the para position, affect the resulting colour of the molecule due to their contributions to the HOMO/LUMO orbital energies. Take a look at the azo-dyes page to view a selection of dyes and their HOMO/LUMO orbital representations.
The transition states for these reactions were found by Henry Rzepa of Imperial college.