Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
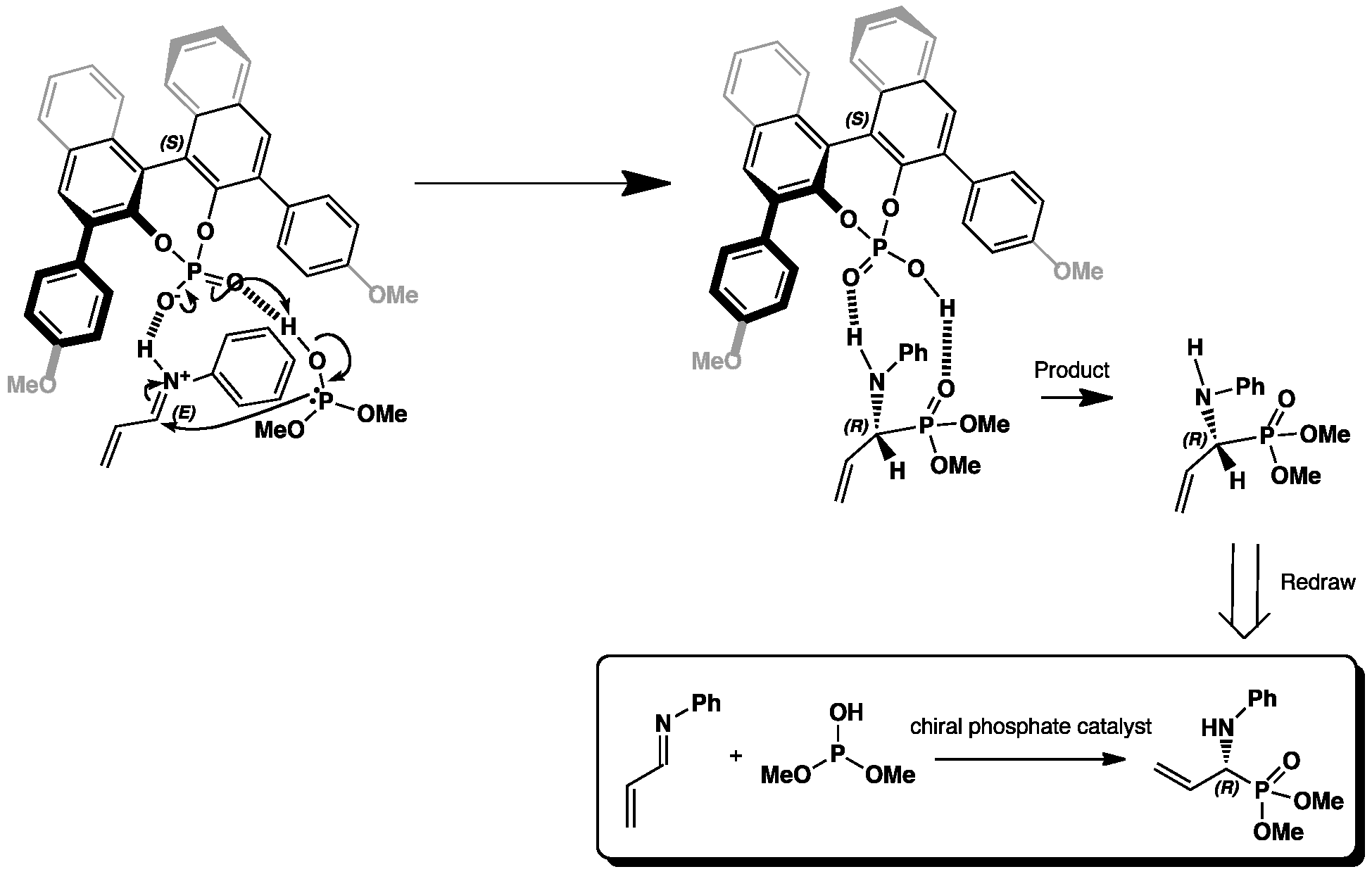
The hydrophosphonylation reaction of an iminium cation with dimethyl hydrogen phosphite with a chiral Brønsted acid phosphate catalyst. The lone pair on the phosphorus of the phosphite attacks the carbon of the iminium to form an amino phosphonate. Because of the large naphthalene groups attached to the phosphate, the catalyst is very rigid and therefore an enantioselective reaction occurs.
The space filled view of this reaction shows the interaction of the oxygen on the chiral phosphate catalyst with the hydrogen of the imine. This effectively holds the nitrogen in place so that only one enantiomer is produced, the R product. Click to view.
M. Yamanaka and T. Hirata, J. Org. Chem., 2009, 74, 3266–3271.