Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
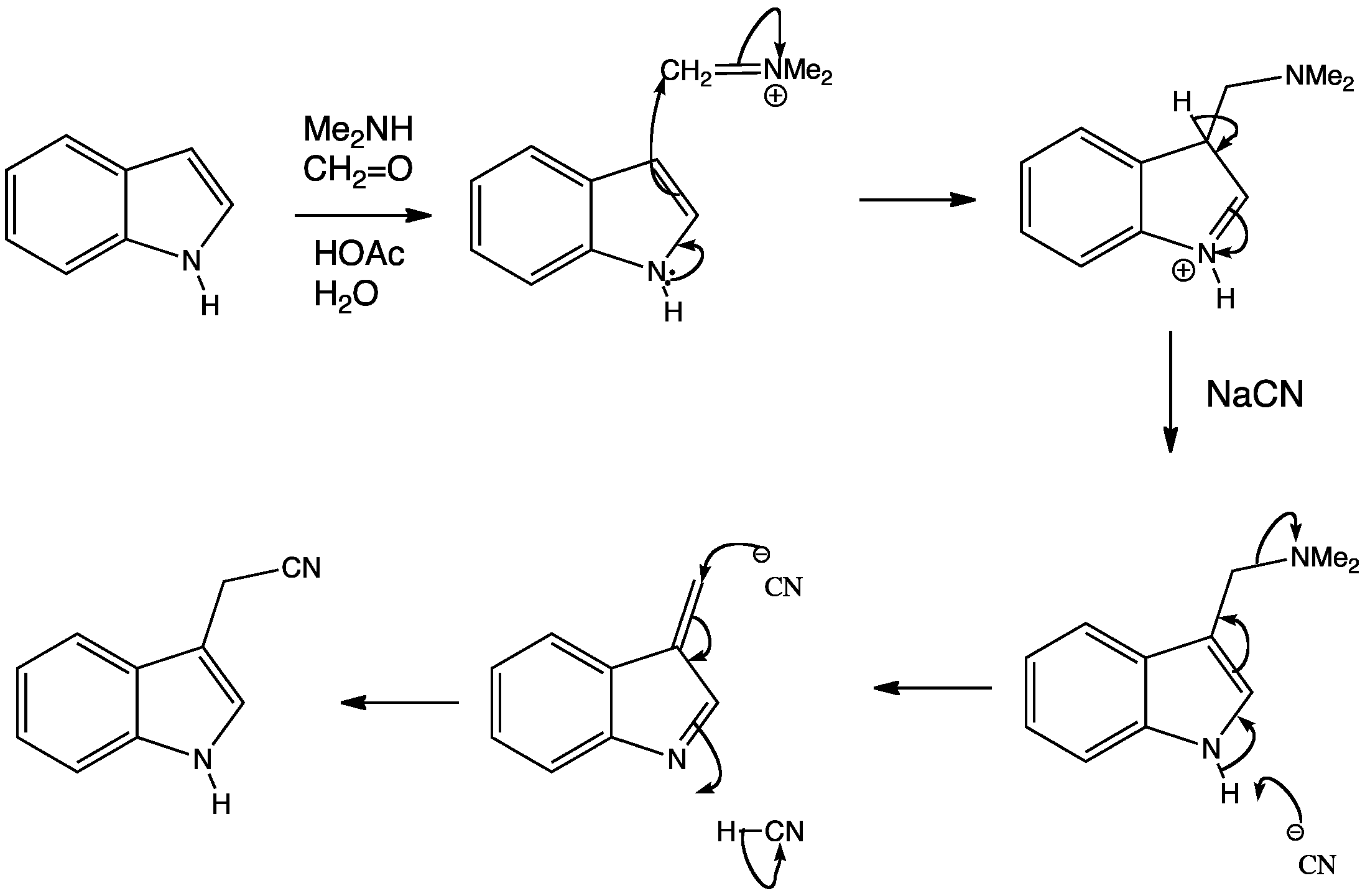
Indole prefers to react mainly through the 3-position. Some reactions go via attack at the 2-position, however this leads to a disruption of the benzene ring and is therefore unfavourable. A good example of a reaction at the 3-position is the Mannich reaction, which works well with pyrrole and furan as well.
The electron donating power of the indole nitrogen is demonstrated well in the following reaction. Normal Mannich bases are often alkylated and eliminated. Alkylation is not needed here as the indole nitrogen can expel the Me2N group when NaCN is used as a base and nucleophile. The result is a substitution by elimination and conjugate addition.
H. R. Snyder, C. W. Smith and J. M. Stewart, J. Am. Chem. Soc., 1944, 66, 200–204.