Click the structures and reaction arrows in sequence to view the 3D models and animations respectively.
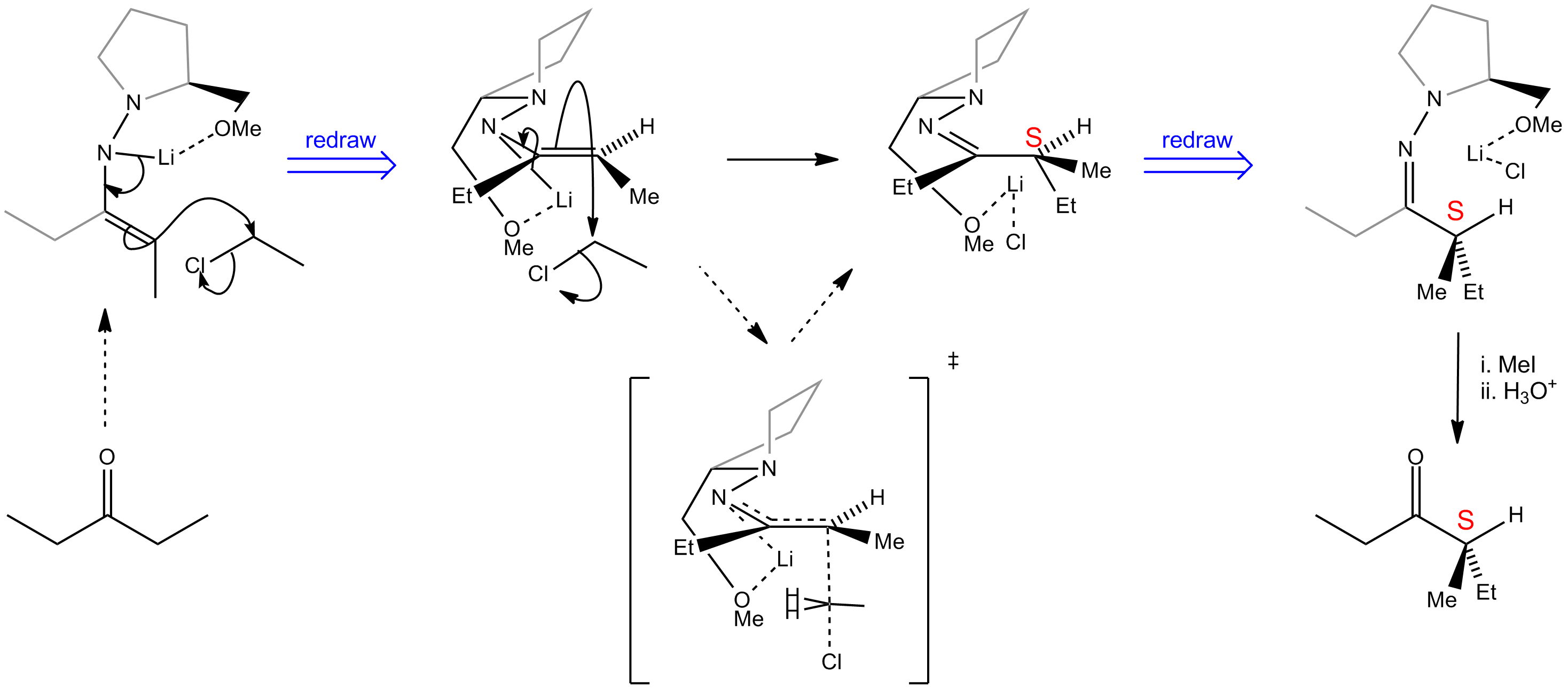
Enantioselective alkylation of ketone enolates can be achieved using SAMP (or the enantiomer RAMP) to form an intermediate hydrazone. The stereochemistry of the reaction is controlled by the chiral auxiliary.
The azaenolate has a rigid, internally chelated structure involving the lithium cation and the methoxy oxygen, making the two faces highly inequivalent. The electrophile then attacks from underneath to give the desired product (S) with high enantiomeric purity. The chiral auxiliary may be removed by quaternisation with methyl iodide followed by hydrolysis.
R. Lazny and A. Nodzewska, Chem. Rev., 2010, 110, 1386–1434.