Click the structures and reaction arrows to view the 3D models and animations respectively
NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
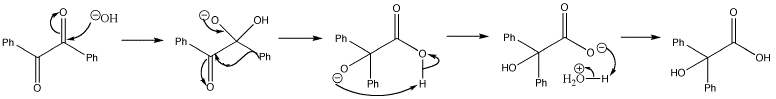
The first rearrangement reaction ever to be described has both the formation of carbonyl groups at the migration origin and destruction of carbonyl groups at the migration terminus. This is known as benzilic acid rearrangement. The mechanism of this benzilic acid rearrangement starts with attack of hydroxide on one of the carbonyl groups. The tetrahedral intermediate can collapse in a reaction reminiscent of a semipinacol rearrangement.
S. Selman and J. F. Eastham, Q. Rev. Chem. Soc., 1960, 14, 221–235.