The Benzene π system has 3 bonding and 3 antibonding molecular orbitals. The strongest interaction is a σ bond between the metal dz2 orbital and the benzene ring. The dxz and dyz orbital form π bonds to the benzene molecule. The next two degenerate levels interact through backbonding from the metal center.
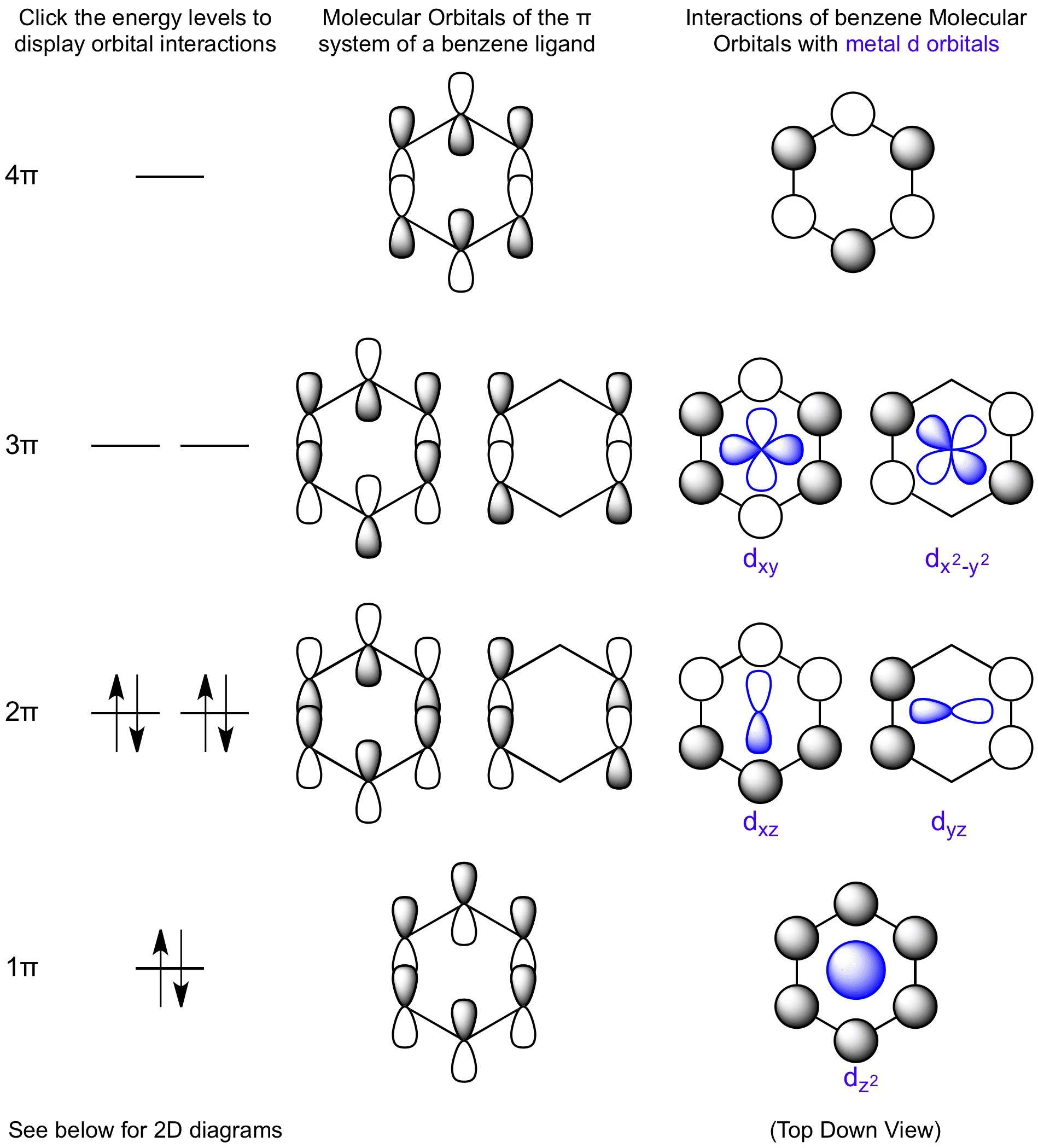
View Benzene Molecular Orbitals here
Explore Metal-Ligand bonding with other molecules
Carbon Monoxide | Phosphine | Hydrogen | Ethylene | Cyclobutadiene | Butadiene | Benzene | Allyl | Cyclopentadienyl | Carbene