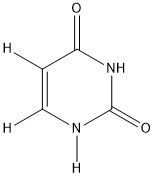
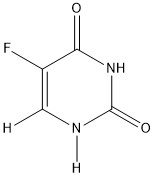
Click the structures to see bond lengths and electron density maps. Red is electron rich, blue is electron poor.
The VDW radius of fluorine (1.35Å) is larger than that of hydrogen (1.10Å), but the fluorine atom is in fact not much larger than that of the hydrogen it has replaced. This means no extra steric demand is added onto the drug molecule and so steric interactions at drug receptor sites are largely unaffected.
The comparison also shows that there is a larger amount of electron density towards fluorine, so it can form strong interactions. This can be useful for drug design.
In the above example 5-fluorouracil acts as a transition state inhibitor and is an example of an isosteric replacement of hydrogen. 5-fluorouracil acts as an antimetabolite that irreversibly inhibits Thymidylate synthetase by competitive binding due to the the carbon to fluorine bond, which is not present in uracil alone. So, 5-fluorouracil can act as an anti-cancer agent.