Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
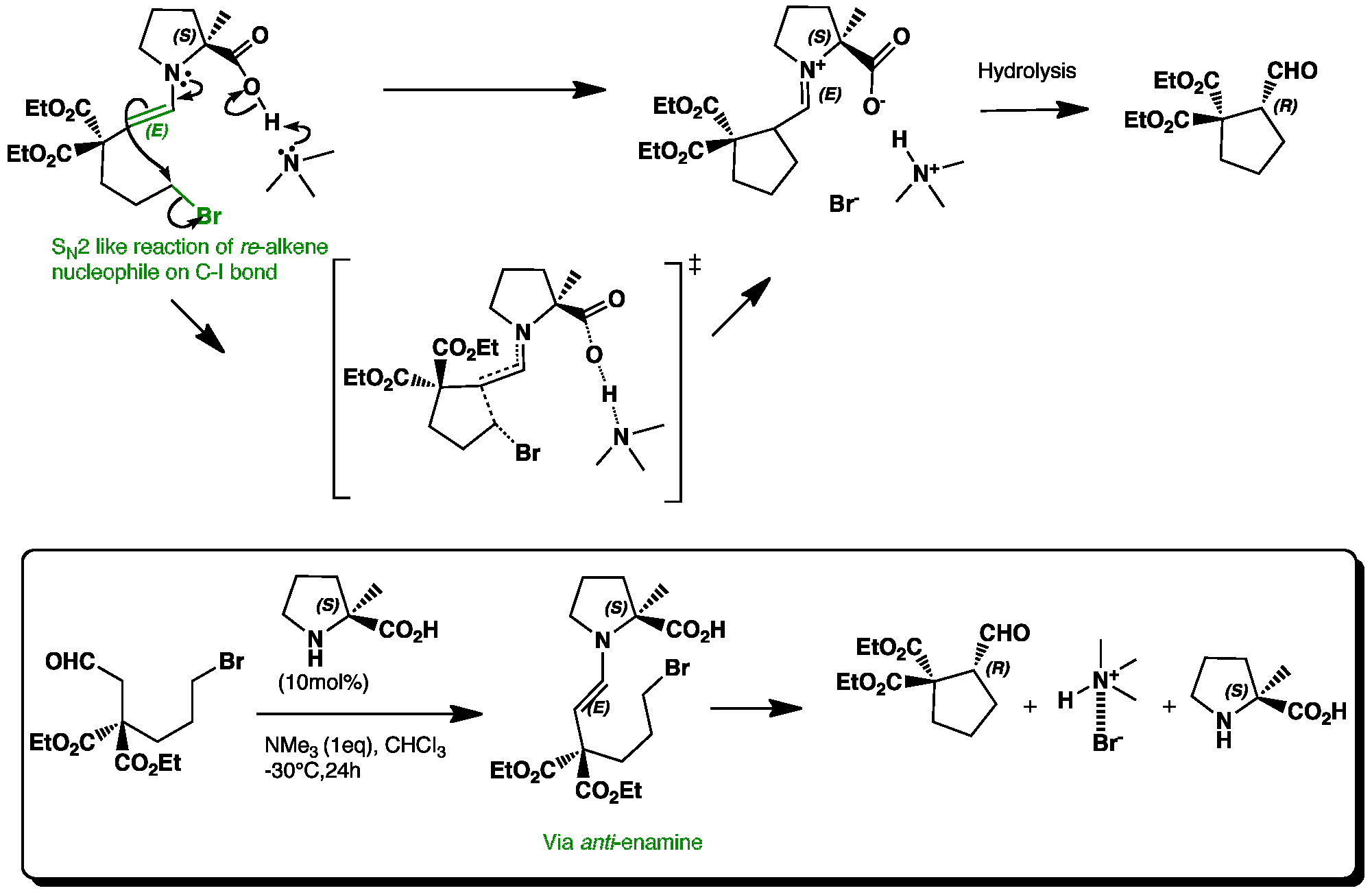
The use of 2-methylproline means that the anti transition state is much lower in energy than the syn transition state, because it has less steric repulsions from the methyl group. The enamine undergoes and intramolecular nucleophilic substitution reaction similar to an SN2 reaction. Formation of the C-C bond and removal of iodide occurs at around 170° with inversion of stereochemistry. Trimethylamine removes the proton from the carboxylic acid group which stabilises the transition state further as the iodide is removed.
A. Fu, B. List and W. Thiel, J. Org. Chem., 2006, 71, 320–326.
J. Liu and L. Wang, Synthesis (Stuttg)., 2016, 49, 960–972.