Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
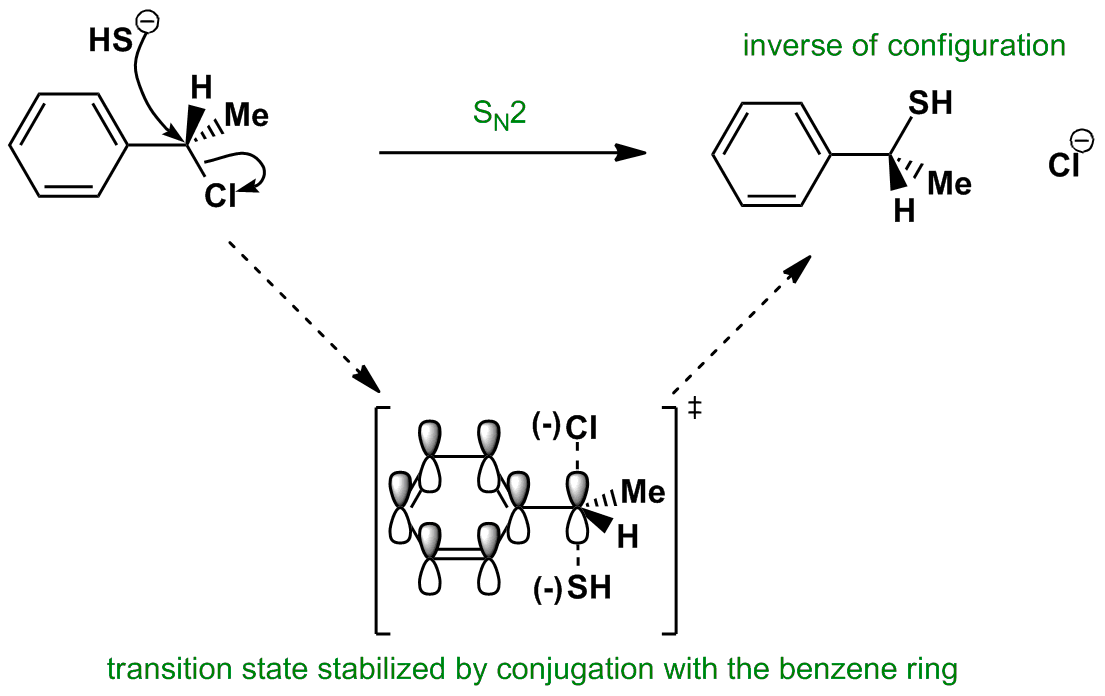
The π framework of p orbitals provides stabilisation in the transition state. This improves the rate of the SN2 reaction and also causes the SN2 reaction to be favoured over SN1. Another key aspect of this reaction is the change of configuration at the carbon – from S to R. This is because the nucleophile attacks from behind the leaving group.
For the Animated Molecular Orbital, only one pi bond has been shown on the phenyl ring for simplicity.
Alternatively, use these links: (Substrate: Nucleophile)
Allyl chloride : SH | Benzyl chloride : SH | 2o benzyl chloride : SH | 2o allyl chloride : SH (SN2′)
R. H. DeWolfe and W. G. Young, Chem. Rev., 1956, 56, 753–901.
J. W. Hill and A. Fry, J. Am. Chem. Soc., 1962, 84, 2763–2769.