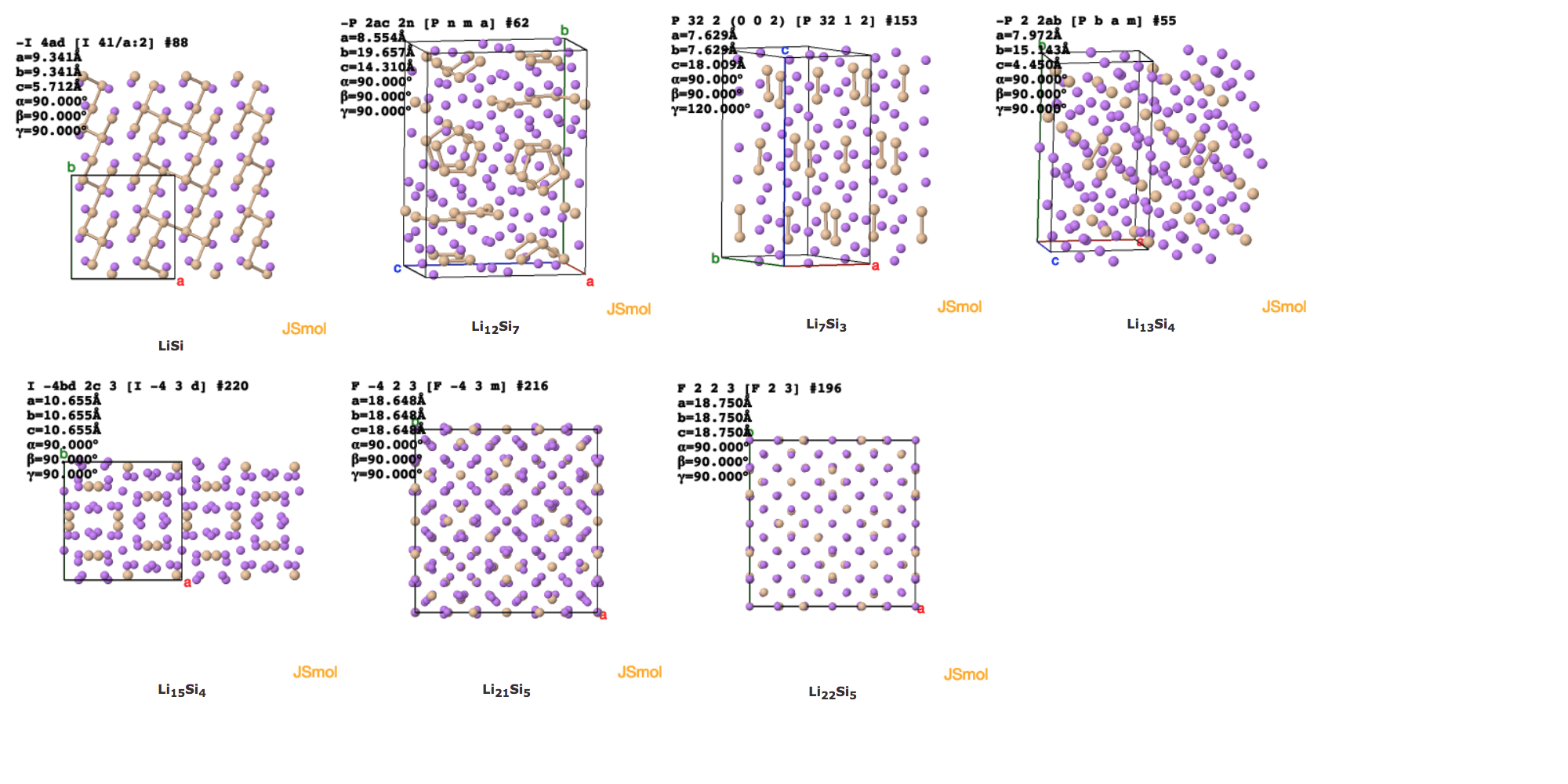
Click the images to see the structures in 3D.
The extent of charging/lithiation of Si increases from left to right: LiSi, Li12Si7, Li7Si3, Li13Si4, Li15Si4, Li21Si5 and Li22Si5.
Lithium forms alloys with silicon in silicon anodes. Silicon has a very high theoretical capacity for lithium insertion, which is more than 10 times that of graphite. However, the conductivity of silicon is low, and it suffers serious volume expansion (more than 300%) during lithium insertion, which results in capacity decay and solid electrolyte interphase damage.
The trend in industry now is to use silicon as an addition to the graphite anodes to improve the capacity while maintaining the reversibility. Tesla’s newest Model 3 car, whose battery is provided by Panasonic, has added about 10% silicon to the graphite anode.
Lithium ions’ insertion into silicon produces different ordered states. Above are the states of LiSi, Li12Si7, Li7Si3, Li13Si4, Li15Si4, Li21Si5 and Li22Si5.
Comparing the models of different lithium insertion states, it can be seen that the average distance between each silicon atom increases as the lithium/silicon ratio increases. This explains the large volume expansion (>300%) of the silicon anode after lithium insertion.
V. L. Chevrier, J. W. Zwanziger and J. R. Dahn, J. Alloys Compd., 2010, 496, 25-36.