Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
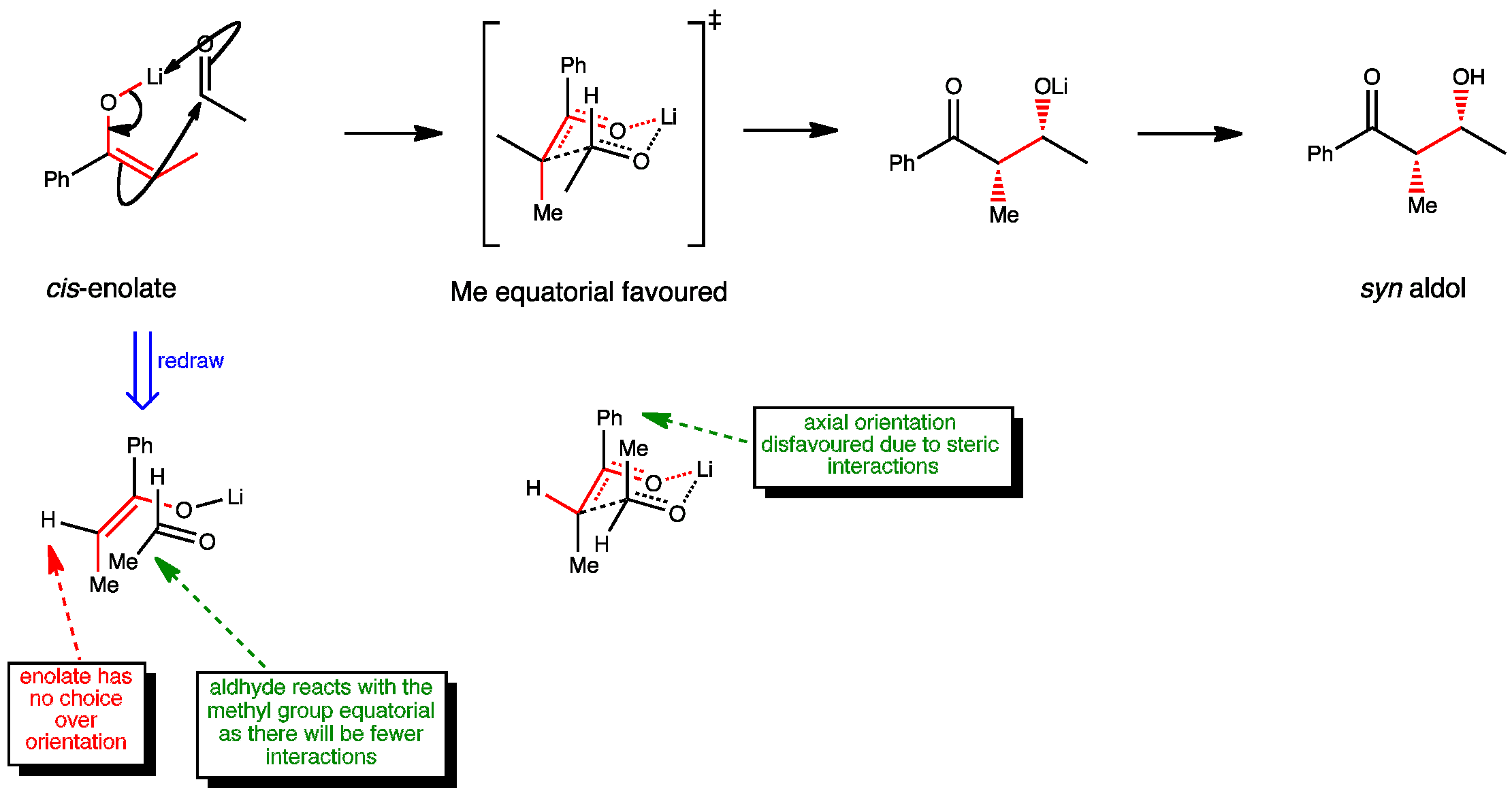
In an aldol reaction, cis enolates prefer to form syn aldols. The reaction proceeds via a chair-like transition state where the aldehyde chooses to react with the methyl group equatorial. As the enolate has no choice over its orientation, only a syn product is possible.