NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition state phase.
Click the structures and reaction black arrows in sequence to view the 3D models and animations respectively
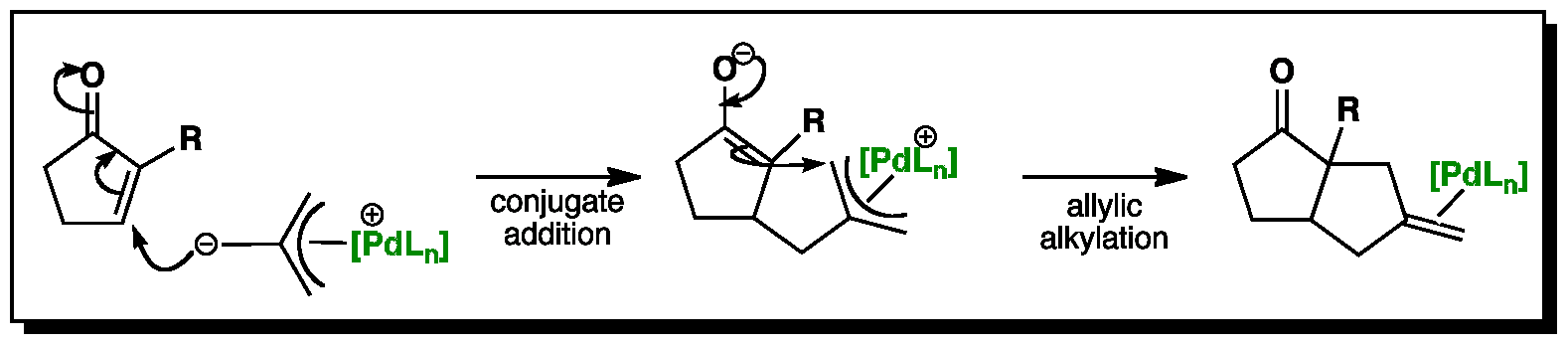
In these animations, the R group is represented as a purple hydrogen. The mechanism is thought to be stepwise whereby the carbanion undergoes a conjugate addition reaction with cyclopentanone. This is followed by an attack on the Pi-allyl palladium unit by the formed enolate. The resulting product from this overall ‘cycloaddition’ has an exomethylene group.