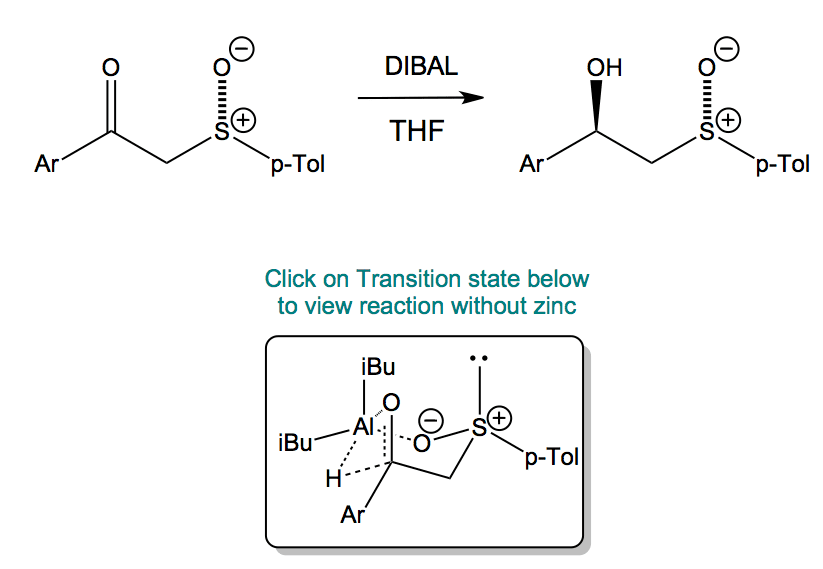
The transition state is a six-membered chair with the toluyl group equatorial. This is the least hindered of the 4 possible transition states.
The stereochemistry of the reaction is controlled by the sulfoxide. In the absence of the ZnCl2, the hydride transfer occurs intramolecularly yielding the R,S enantiomer.
Summary page : Without ZnCl2 : With ZnCl2
A. Solladié-Cavallo, J. Suffert, A. Adib and G. Solladié, Tetrahedron Lett., 1990, 31, 6649–6652.