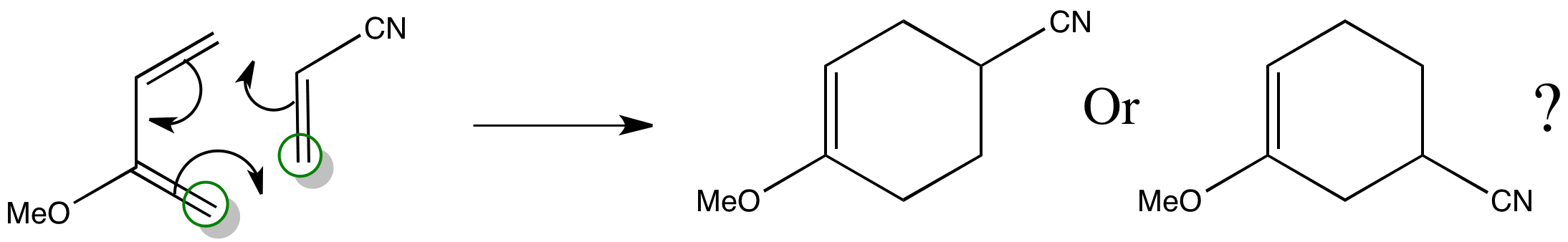
It is possible to tell which product is formed by considering the molecular orbitals of the reactants.
The regioselectivity of the reaction can be determined by working out where the largest coefficient of the HOMO lies in the diene and the largest coefficient of the LUMO lies in the dienophile. The reagents come together in the orientation where these ‘largest’ coefficients can overlap.
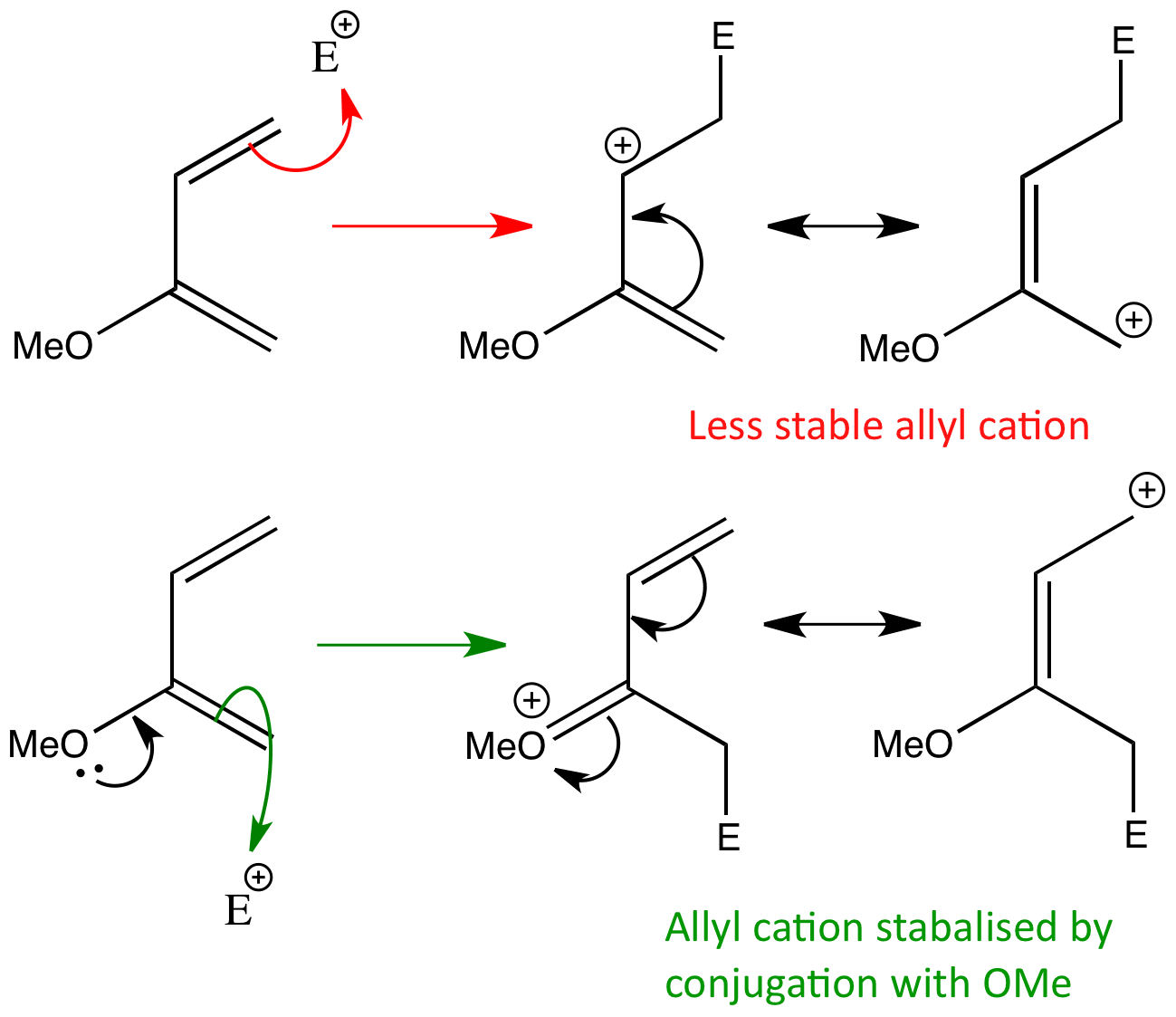
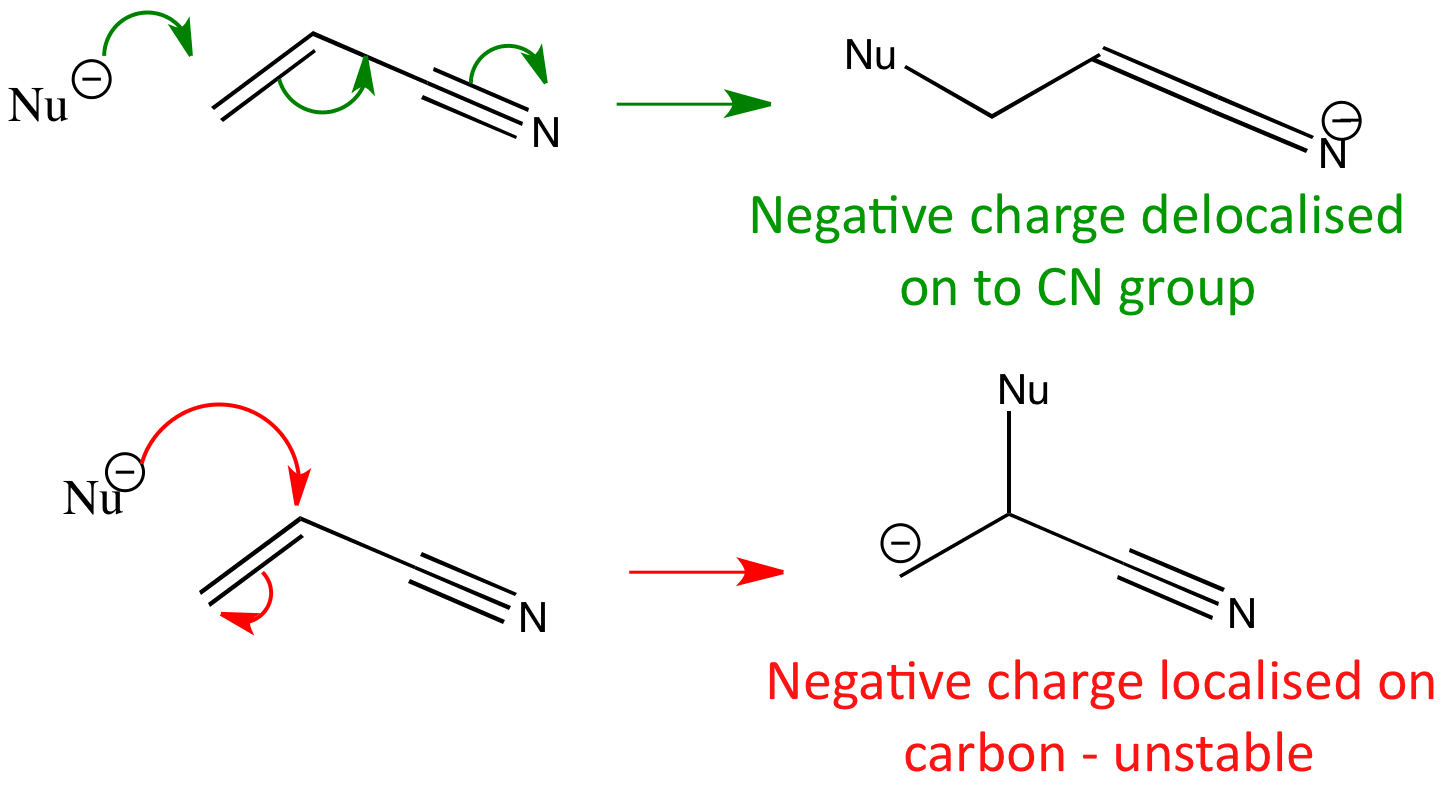
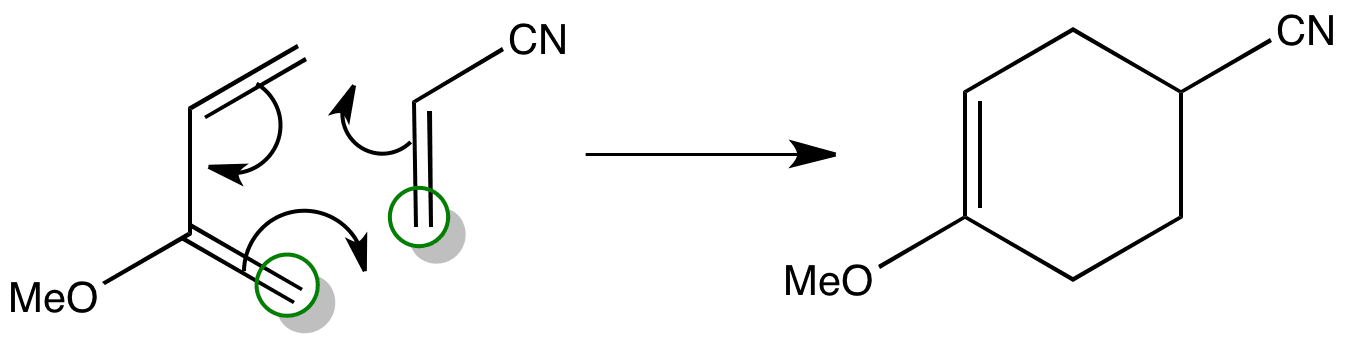
Combining the two ends gives the product that is formed.
J. Sauer, Angew. Chemie Int. Ed. English, 1967, 6, 16–33.