Alpha silver iodide is a form of silver iodided that forms above 420K. The animation shown uses a simplified version of the sturucture. The conductivity occurs by the Ag+ ions moving in and out of their interstial sites. For more detail on the 3D structure click here.
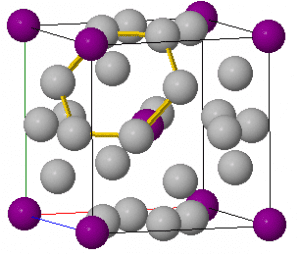
This can occur with all the Ag+ ions with the button labelled”Show conduction pathways” demonstrating how the silver ions throughout the sturcutre can move.
Alpha-AgI is known as a fast ion conductor. Fast ion conductors have many potential uses for example developments for solid electrolytes in batteries.
To return to the ionic conductivity home page click here.
Nield, D. Keen, W. Hayes and R. McGreevy, Solid State Ionics, 1993, 66, 247-258.