Alpha silver iodide is a material that exhibits ionic conduction. This is due to its struture. The Ag + ions can occupy 42 different sites. The different buttons show the different number of potential sites. The randomized structure shows how the siliver ions can be arranged randomly as well as displaying the prefrence order of Tetrahedtral > trigonal > Octrahedral sites.
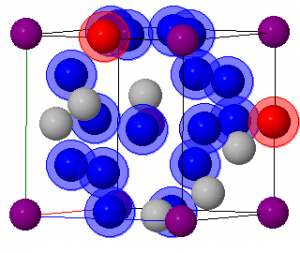
Each button shows each of the sites individually. There are 2 buttons to show the trigonal sites that can be occupied as these are numerous.
In the random struture tetrahedral are blue and octahedral sites are in red. The trigonal sites left silver.
To return to the ionic conductivity home page click here.
D. A. Keen, J. Phys.: Condens. Matter, 2002, 14, R819-R857.