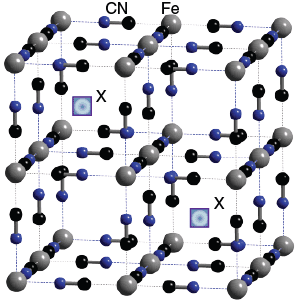
The Fe(III) and Fe (II) are linked in a cubic shape by CN– ions. The sites labelled ‘X’ in the diagram highlight regions that cations or water may occupy.
The low spin Fe(II) ions are linked in an octahedron to 6 carbons, whilst the high spin Fe(III) ions are linked in an octahedron to 6 nitrogens.