Click Image to Display Alternate Structure
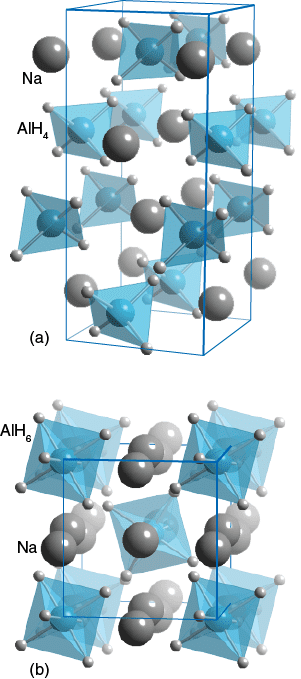
The hydride coordination around Aluminium differs between these two compunds. In (a) NaAlH4 it is a 4 coordinate tetrahedron; whilst in (b) Na3AlH6 it is a 6 coordinate octahedron. Both these compounds have hydrogen storage capabilities.