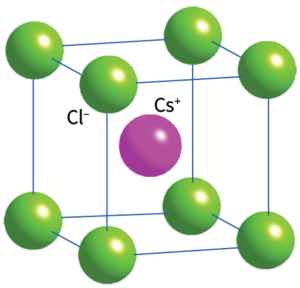
– 8:8 (cubic)
Non close-packed, arrangement of Cl with Cs in cubic holes.
Polyhedra – Face-sharing and
cubes
All of the cubic holes in a simple cubic packing are occupied.
Both ions show cubic coordination (CN = 8)
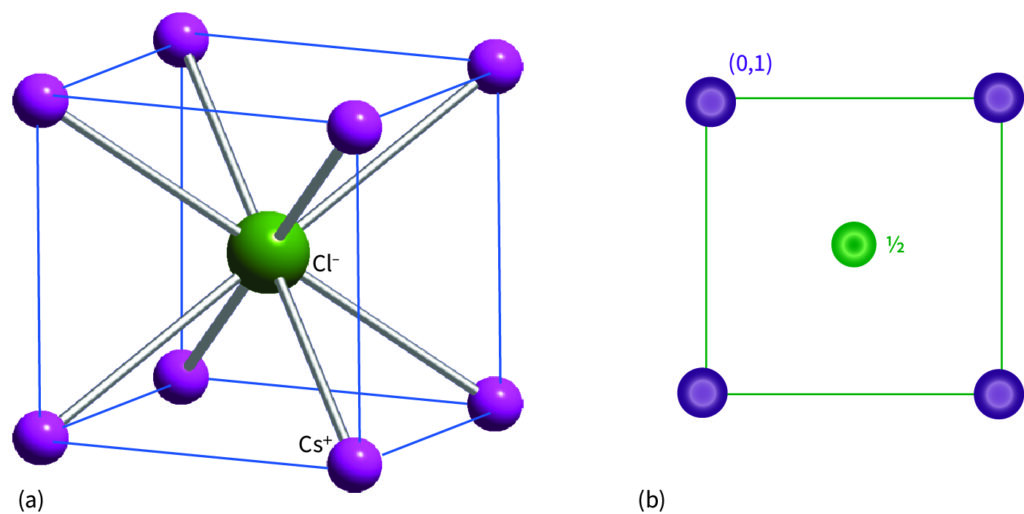
(a) The caesium-chloride structure. The corner lattice points, which are shared by eight neighbouring cells, are surrounded by eight nearest-neighbour lattice points. The anion occupies a cubic hole in a primitive cubic lattice; (b) its projection.
The CsCl structure adopted by CsCl, CsBr, and CsI under normal conditions.
Related Structure: Body-centred cubic (bcc)