NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
Click the structures and reaction arrows to view the 3D models and animations respectively
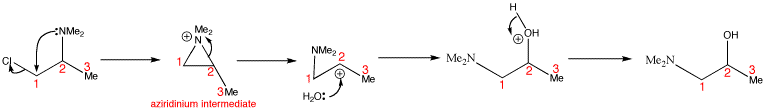
Water opens the aziridinium intermediate at the more hindered end, so the amine migrates from the secondary to the primary position. Water is a weak nucleophile and therefore cannot undergo SN2 reactions. Secondary centres can undergo either SN1 or SN2 reactions. Water waits until the leaving group has left of its own accord, to give a cation, which rapidly grabs any nucleophile. This can only happen at the secondary centre as the primary cation is too unstable and cannot form.