NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition phase
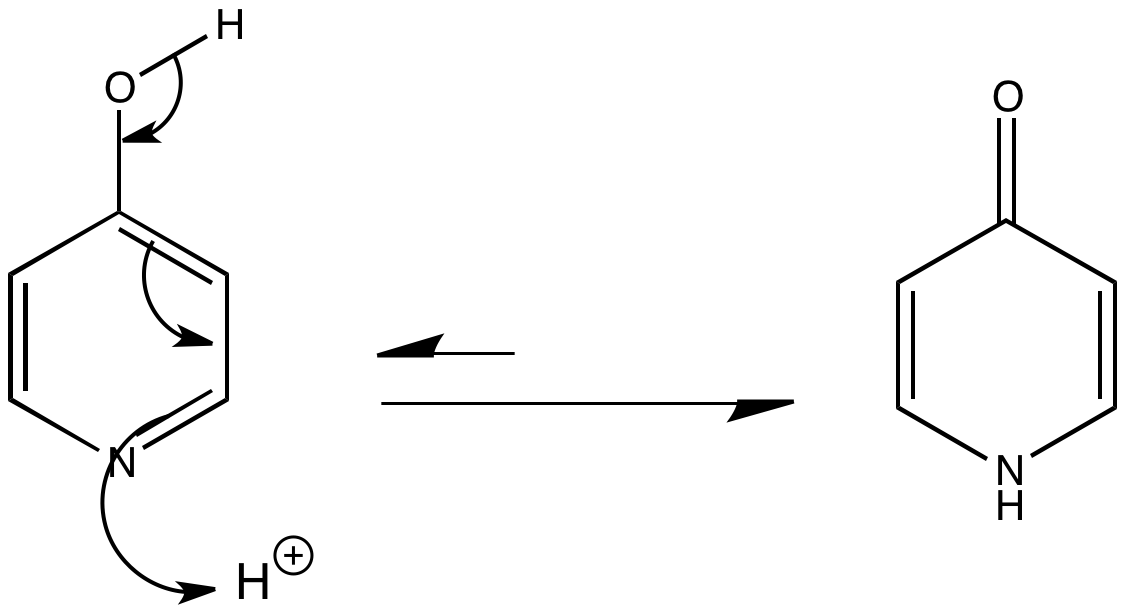
4-Hydroxypyridine undergoes tautomerism to give 4-pyridone (a carbonyl compound). Pyridones are still aromatic as the lone pair of electrons on nitrogen can be delocalised into the ring.
Intermolecular hydrogen bonding both in solution and solid state favours the pyridone form.