A Frenkel defect is a point defect. This occurs when the cation moves into an interstital site that is usually unoccupied. In the animation, silver chloride is used to demonstrate a Frenkel defect. An Ag+ ion moves into the tetrahedral hole that is unoccupied.
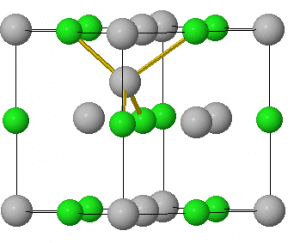
Frenkel defects, unlike Schottky defects, do not change volume as mass and density has remained the same.
Frenkel defects are intrinsic defects – these are defects that can occur naturally. They occur naturally because moving into an interstital site raises the entropy of the system and thus lowers the Gibbs energy making this movement favourable.
There is an energy barrier to doing this however, and heat is required to overcome this barrier. The result of this is the number of defects is related to the temperature of the solid – the greater the temperature the more defects that occur.
Return to ionic conductivity home page.
Y.V.G.S. Murti and R. D. Banhatti, Bull. Mater. Sci., 1997, 20, 435-440.