Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
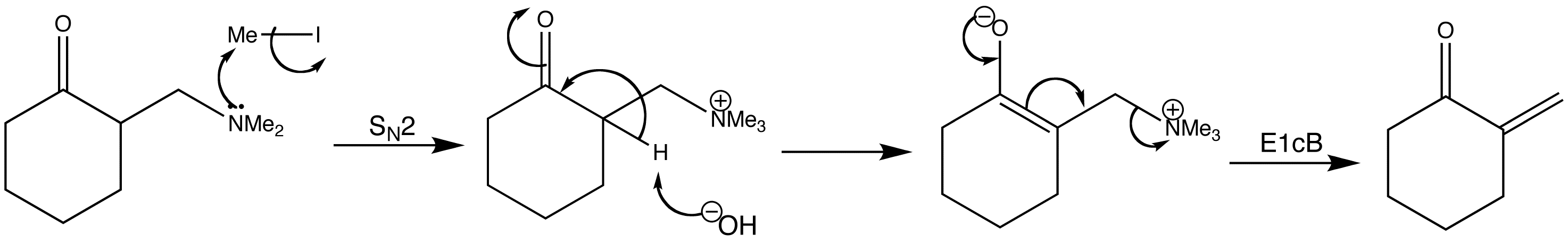
The Mannich products can be converted to enones. The most reliable method for making the enone is to alkylate the Mannich base with MeI and then treat the ammonium salt with base. Enolate ion formation leads to an E1cB reaction rather like the dehydration of aldols, but with a better leaving group.
T. F. Cummings and J. R. Shelton, J. Org. Chem, 1960, 25, 419–423.
H. G. O. Alvim, E. N. da Silva Júnior and B. A. D. Neto, RSC Adv., 2014, 4, 54282–54299.