Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
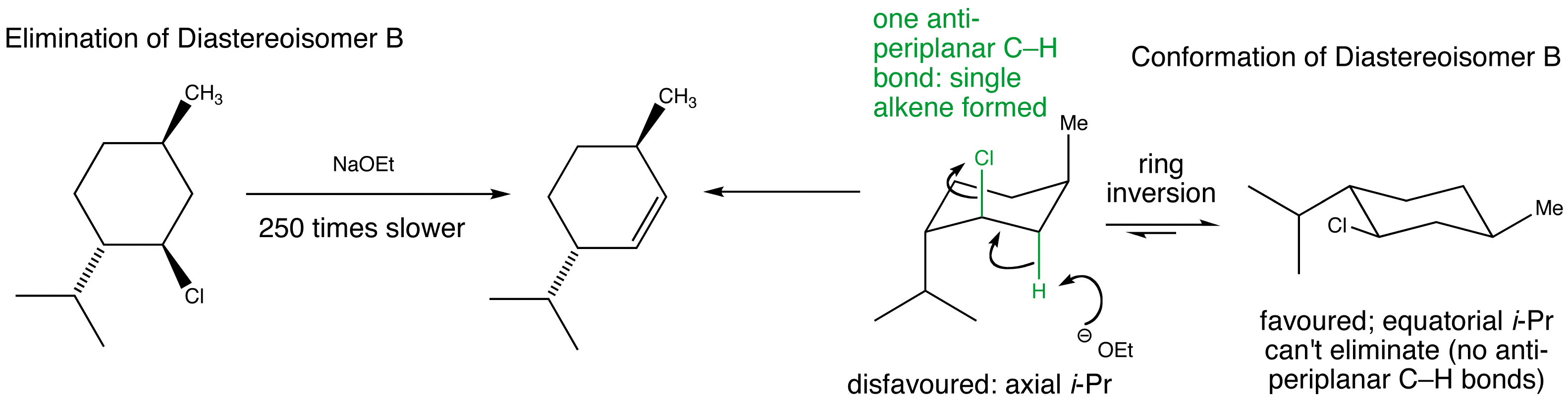
Stereospecific E2 eliminations – two diastereoisomeric chlorides derived from menthol give two different regioisomers of alkene.
Manipulate the conformations to explore the arrangements of the protons and the chlorine. Are they anti-periplanar as required for E2?
Examine the animated energy plots of Diastereoisomer A and Diastereoisomer B to determine the relative energies of the anti-periplanar conformations required for E2 elimination. Observe the very high energy all-axial conformation and how this is avoided by using a twist-boat conformation in the elimination mechanism.
J. F. Bunnett, Angew. Chemie Int. Ed. English, 1962, 1, 225–235.