Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
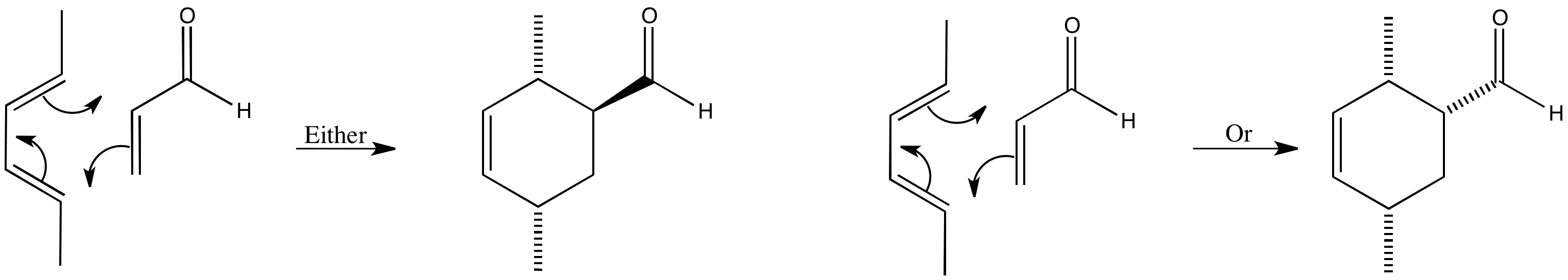
In this case both the diene and dienophile contribute to the stereochemistry of the product. Although there are three stereogenic centres in the product only two diastereoisomers can be formed. The diastereoisomers formed are known as exo and endo.
The endo product is favoured, but which one is it? The easiest way to work this out is by drawing the reactants coming together in 3D. See one method for working this out.