Please note, due to the complexity of the structure this page may take longer to load
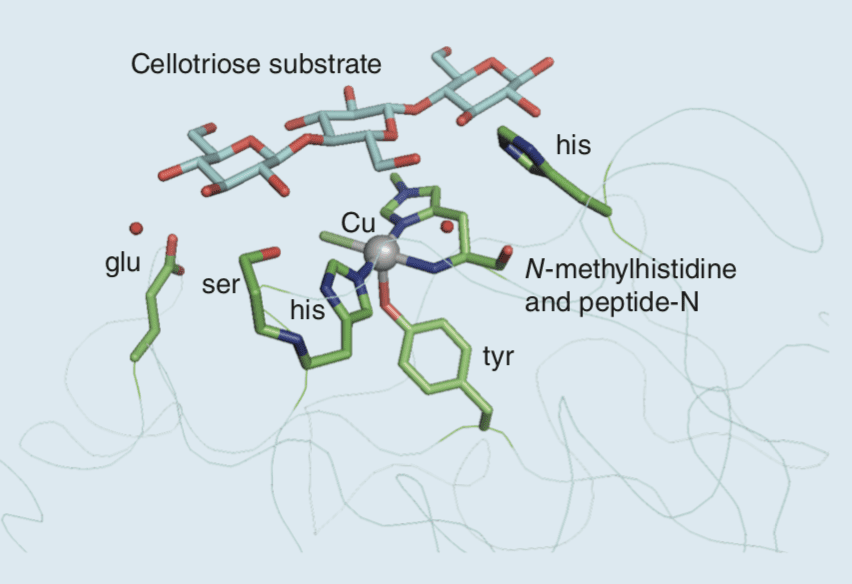
The crystal structure of a lytic polysaccharide monooxygenases (LPMO) in the presence of a cellotriose, a small water-soluble substrate. The Cu is located close to the surface of the enzyme: it is coordinated by an N-methylhistidine that lies at the N-terminus, along with its peptide-N, a second histidine, and a tyrosine.
Equatorial coordination is completed by a chloride ion which indicates the position at which an O2 molecule is likely to be coordinated during the catalytic cycle. The cellobiose molecule is tethered by several polar contacts with amino acid side chains and structured water molecules that position the target C−H bond close to the position occupied by the Cl ligand.