Step 2 – Enantioselective Addition
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
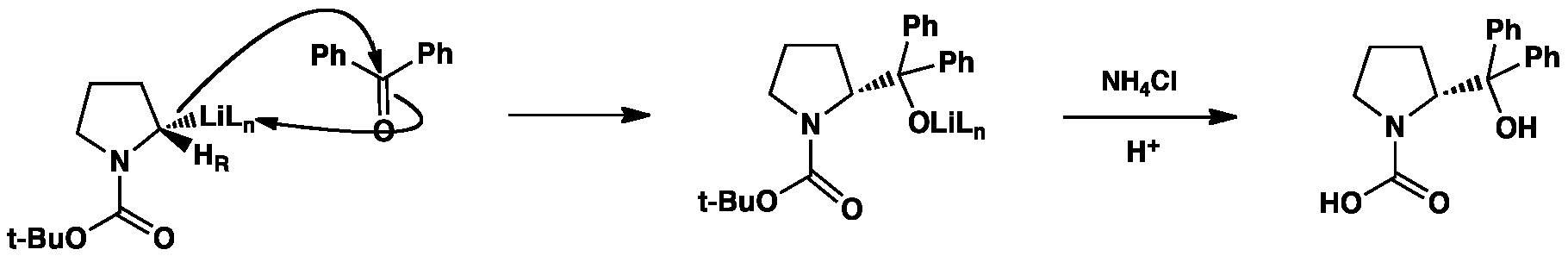
The stereodefined alkyllithium can then attack an electrophile (benzophenone in this example) to give, after hydrolysis, an enantiomerically enriched product.
K. B. Wiberg and W. F. Bailey, J. Am. Chem. Soc., 2001, 123, 8231–8238.