NOTE: Important charges and non-bonding electrons are shown throughout the animation except during the transition state phase.
Click the structures and reaction black arrows in sequence to view the 3D models and animations respectively
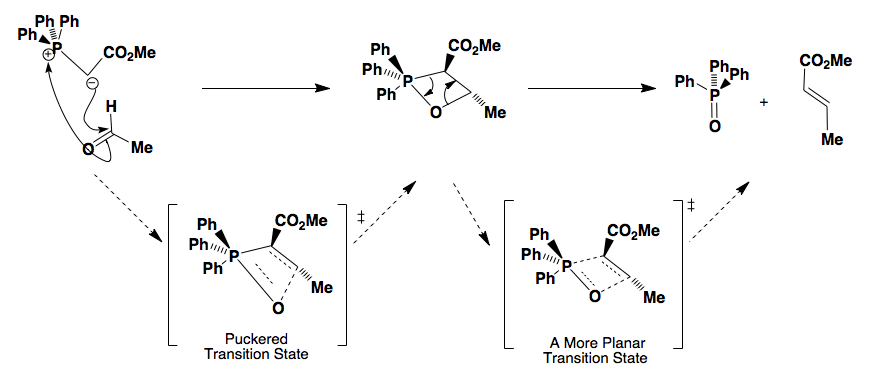
The first step of the E-selective Wittig reaction
is the production of the anti oxaphosphetane. The difference between this process and the Z-selective Wittig reaction lies in the repulsion between the aldehyde C=O and the stabilising substituent on the ylid (CO2Me) forcing the transition state into the anti conformation, preventing the high puckering of the transition state as in the Z-selective Wittig reaction.
The second stage is the decomposition of the oxaphosphetane intermediate. After a phosphorous pseudorotation,
the intermediate decomposes via a concerted transition state to form the E-alkene and the phosphine oxide.