Symbols, Terminology and Constants in Science and Mathematics
Science and Mathematics uses a large range of units, symbols and terms, and initially it can seem like a foreign language. Here we have an inexhaustive list of commonly used mathematical terms and symbols that appear commonly in all sciences – especially physical and inorganic chemistry, spectroscopy and analytical chemistry. Click on these links to jump to The Greek Alphabet – Factor Prefixes – Constants – Formulae
The modern alphabet on which most Western alphabets are based is a combination of the Latin and Greek alphabets. Characters from the Greek alphabet appear in most science and mathematics. They can be used to represent more than one quantity. Usually the use is specified. However, some common occurrences are listed below. NOTE: (upper) and (lower) indicate the upper case and lower case characters respectively.
| Lower case | Upper case | Name | Common use |
| α | Α | alpha | Used as a general variable in numerous situations. Always specified. Used often in quantum mechanics. |
| β | Β | beta | Used as a general variable in numerous situations. Always specified. used often in quantum mechanics. |
| γ | Γ | gamma | An irrep in group theory (upper). |
| δ | Δ | delta | Indicates an infinitesimal change (lower) or general finite change (upper). Lower case is also used to signify a partial derivative. |
| ε | Ε | epsilon | Permitivity of free space (ε0). |
| ζ | Ζ | zeta | Effective nuclear charge in quantum chemistry (lower). |
| η | Η | eta | Used to represent many coefficients. Commonly, viscosity (lower). |
| θ | Θ | theta | A context-specific angle (lower), or heat (upper). |
| ι | Ι | iota | |
| κ | Κ | kappa | Numerous uses. In chemistry – the compressability of a compound (lower). |
| λ | Λ | lambda | Wavelength (lower) of a wave or particle. |
| μ | Μ | mu | Symbol for “micro” (1×10-6) (lower). Also the symbol for “reduced mass” – see below. |
| ν | Ν | nu | Frequency, Hz or s-1 (lower) |
| ξ | Ξ | xi | Numerous uses. Used instead of Q for the partition function in a grand canonical ensemble in statistical mechanics (upper). |
| ο | Ο | omicron | |
| π | Π | pi | Important irrational number in mathematics (lower). Mathematical operator for “product of all terms over a specified region” (upper). |
| ρ | Ρ | rho | Density (lower). Can be physical density (kg m-3), or some specified density, such as electron density (ρ(e–)). |
| σ | Σ | sigma | Numerous uses. Indicates sigma-bonds, collision cross-section etc. (lower). Mathematical operator for “sum of all terms over a specified region” (upper). |
| τ | Τ | tau | Some specific time not denoted by t (lower). Usually the “1/e” time. |
| υ | Υ | upsilon | Uncommonly used. Usage usually specified. |
| φ | Φ | phi | Numerous uses in the mathematics of quantum mechanics. Can indicate a specific eigenstate (lower), a general eigenstate (upper). Used in geometry to indicate an angle in the spherical polar co-ordinate system (lower). |
| χ | Χ | chi | The character of an irrep in group theory (upper). |
| ψ | Ψ | psi | Used extensively in quantum mechanics. Represents wavefunctions. Component of the Schrödinger equation. Lower case is usually a specific wavefunction, whereas upper case might be a general wavefunction. |
| ω | Ω | omega | Angular frequency (lower) in rotation. Unit of electrical resistance (upper). Can be adapted to be used in rotation-vibration spectroscopy (e.g ϖ, the central point between the P and Q branches in rot-vib spec.) |
Often – rather than using a number in standard mathematical form -it is preferable to use a quantity’s units, with a suitable factor prefix to indicate scale. For example, it is much quicker and more simple to write λ = 4.00×10-7 m as 400 nm. The n, for “nano”, is a prefix used to indicate a factor of 1×10-9.
| Prefix | Name | Scale |
| E | exa | 1018 |
| P | peta | 1015 |
| T | tera | 1012 |
| G | giga | 109 |
| M | mega | 106 |
| k | kilo | 103 |
| d | deci | 101 |
| c | centi | 10-2 |
| m | milli | 10-3 |
| μ | micro | 10-6 |
| n | nano | 10-9 |
| p | pico | 10-12 |
| f | femto | 10-15 |
| a | atto | 10-18 |
| z | zepto | 10-21 |
| y | yocto | 10-24 |
Below are listed some useful constants that are important in physical chemistry and spectroscopy.
| Constant | Value | Units | Equivalency |
| h – Planck’s constant | 6.626068×10-34 | J s | |
| ħ – reduced Planck’s constant | 1.054571×10-34 | J s | h / 2π |
| amu – atomic mass unit | 1.660539×10-27 | kg | |
| kB – Boltzmann’s constant | 1.380650×10-23 | J K-1 | |
| R – molar gas constant | 8.314472×100 | J mol-1 K-1 | NAkB |
| NA – Avogadro’s number | 6.022141×1023 | (atoms, particles etc.) mol-1 | |
| c – speed of light in a vacuum | 2.997925×108 | m s-1 | |
| me – mass of an electron | 9.109382×10-31 | kg | |
| e – elementary charge | 1.602177×10-19 | C | |
| ε0 – permittivity of free space | 8.854188×10-12 | F m-1 | 1 / μ0c02 |
| μ0 – vacuum permeability | 4π×10-7 | J s2 / C2 m |
Below are listed some common formulae used in the theory behind basic spectroscopic techniques, divided into relevant subsections.
Basic Equations
Momentum: p = mv
Kinetic Energy: Ek = ½mv2 = p2/2m
Speed of light: c = ν×λ
Energy of a wave: E = hν
de Broglie formula: λ = h/p
Boltzmann Statistics
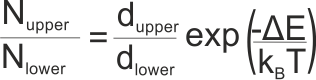
where Nupper is the population of the upper state, Nlower is the population of the lower state, dupper and dlower are the degeneracies of the upper and lower states, ΔE is the energy gap between the two states (in Joules, not kJ), kB is Boltzmann’s constant and T is the absolute temperature of the system (in K)
Rotational Spectroscopy
![]()
where F(J) is the energy of rotational level J and B is the rotational constant
![]()
where h is Planck’s constant, I is the moment of inertia, c is the speed of light in vacuo, in cm s-1
![]()
where μ is the reduced mass of the molecule
![]()
where degeneracy of a rotational energy level J is given by
![]()
The primary selection rule for pure rotational spectroscopy is
![]()
The line spacing in a pure rotation spectrum is simply given by
![]()
Vibrational Spectroscopy
The energy of a level in a vibrational system is given by
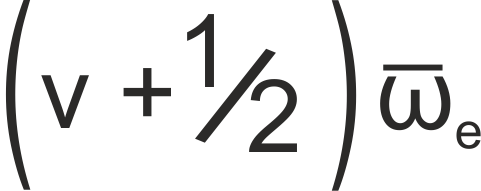
where ωe is the harmonic band centre, given by
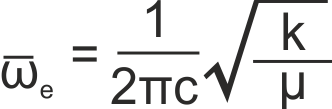
where k is the bond force constant, μ is the reduced mass of the molecule and c is the speed of light in a vacuum. The usual selection rule of Δv = ±1 applies.

