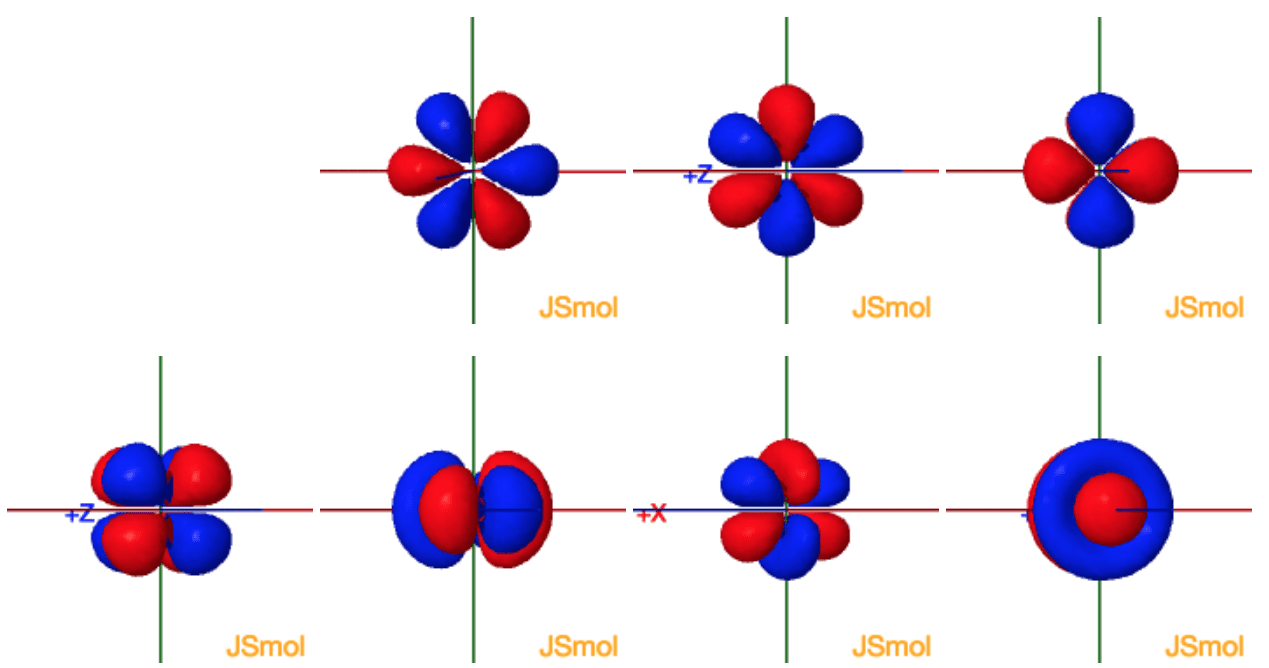
Click the images to see the various 4f orbitals
The lanthanide series is defined by the progressive filling of the 4f orbitals. These seven orbitals have the following ml values:
ml=0, ±1, ±2,
±3
ml=0 are real, all the others are complex and so linear combinations must be taken to obtain
real orbitals.
General set
These are produced by simply taking linear
combinations of ml=±1, ±2, ±3
| ml | |
| 0 | z3 |
| ±1 | xz2 yz2 |
| ±2 | xyz z(x2-y2) |
| ±3 | x(x2-3y2) y(3x2-y2) |
s-orbitals |2p-orbitals |3p-orbitals | 3d-orbitals | 4f-orbitals