Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
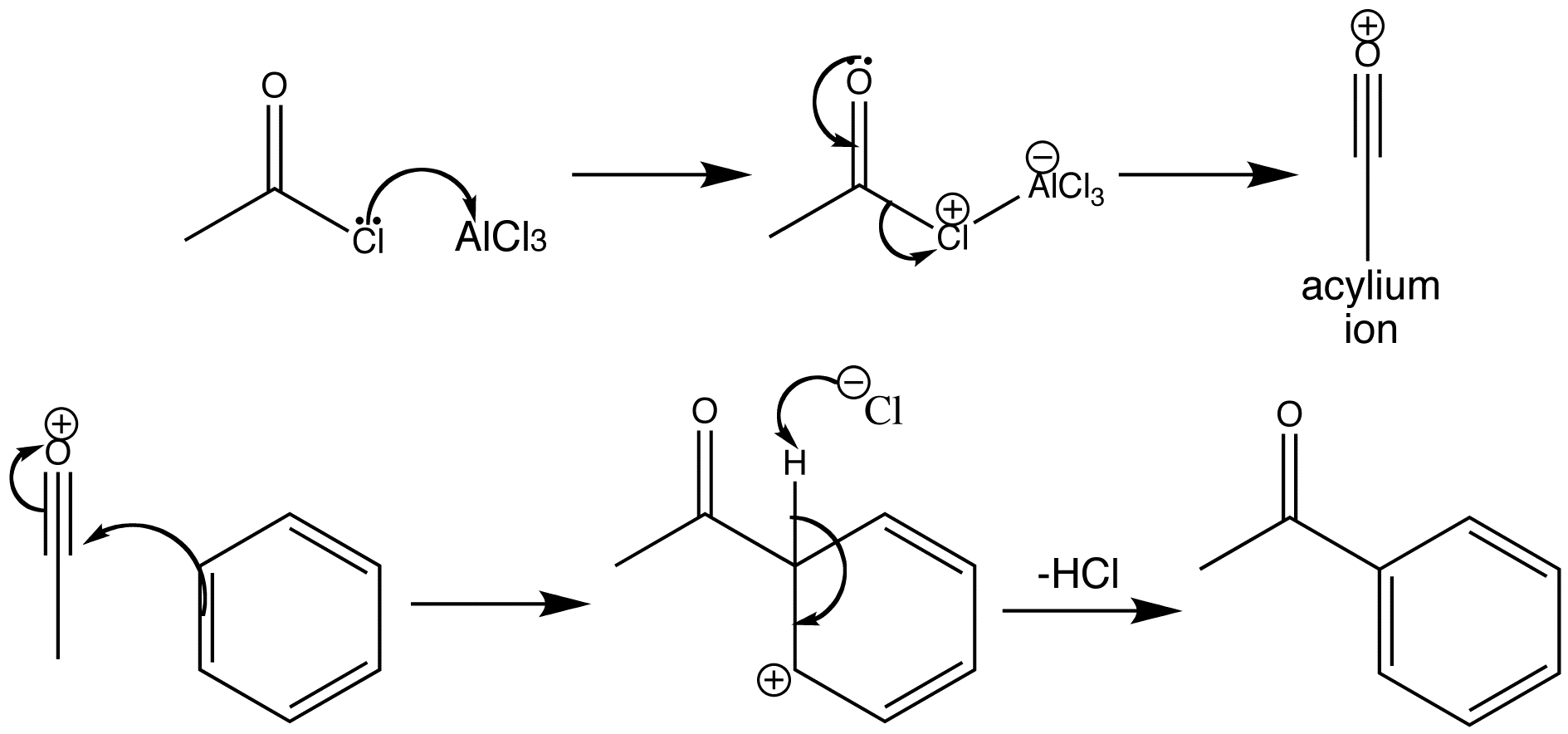
Friedel-Crafts acylation occurs with acid chlorides and AlCl3. Acid chlorides can give the rather stable acylium ion even in hydrolytic reactions and they do so readily with Lewis acid catalysis. Attack on a benzene ring then gives an aromatic ketone. The benzene ring has been acylated.