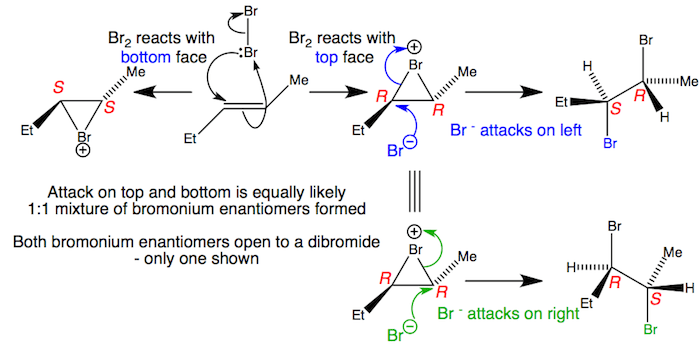
Unsymmetrical bromonium ions open to give racemates
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
Alkenes react on both faces to produce a 1:1 mixture of enantiomeric bromonium ions. Each of these bromonium ions can be opened at both ends by the liberated bromide with inversion. The racemic mixtures of bromonium ions produces a racemic mixture of dibromides. Significantly unsymmetrical bromonium ions may display a preference for opening at one end rather than the other but this example will be close to 1:1.