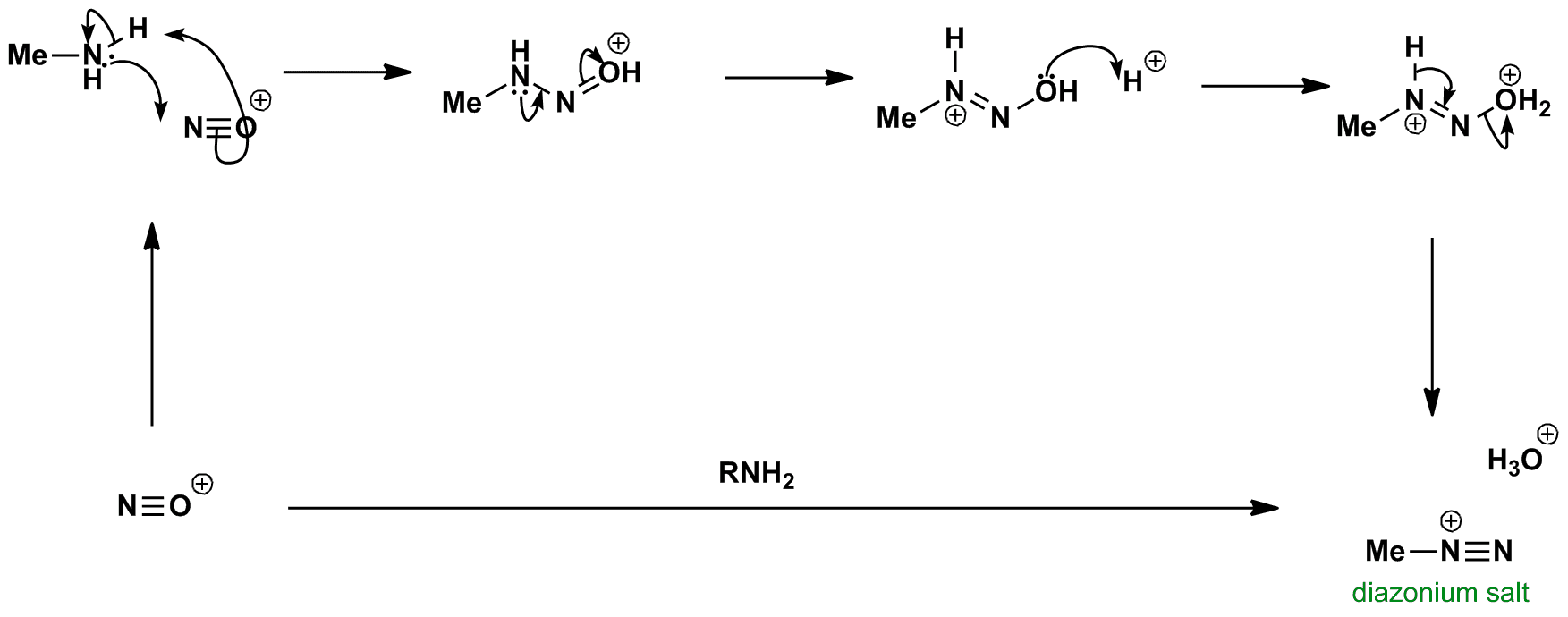
Click the structures and reaction arrows in sequence to view the 3D models and animations respectively
Reaction of amine with the nitrosonium ion gives a diazonium salt. The diazonium salt is usually made in situ since it is usually unstable. If the alkyl group is replaced with an aryl group, the salt is stable at 0 oC and reacts with various nucleophiles.
The diazonium salt is used in Tiffeneau-Demjanov rearrangements.
F. Mo, G. Dong, Y. Zhang and J. Wang, Org. Biomol. Chem., 2013, 11, 1582.